Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 April 2024

Complexity of avian evolution revealed by family-level genomes
- Josefin Stiller ORCID: orcid.org/0000-0001-6009-9581 1 ,
- Shaohong Feng ORCID: orcid.org/0000-0002-2462-7348 2 , 3 , 4 ,
- Al-Aabid Chowdhury 5 ,
- Iker Rivas-González ORCID: orcid.org/0000-0002-0515-0628 6 ,
- David A. Duchêne ORCID: orcid.org/0000-0002-5479-1974 7 ,
- Qi Fang ORCID: orcid.org/0000-0002-9181-8689 8 ,
- Yuan Deng 8 , 9 ,
- Alexey Kozlov ORCID: orcid.org/0000-0001-7394-2718 10 ,
- Alexandros Stamatakis ORCID: orcid.org/0000-0003-0353-0691 10 , 11 , 12 ,
- Santiago Claramunt ORCID: orcid.org/0000-0002-8926-5974 13 , 14 ,
- Jacqueline M. T. Nguyen ORCID: orcid.org/0000-0002-3076-0006 15 , 16 ,
- Simon Y. W. Ho ORCID: orcid.org/0000-0002-0361-2307 5 ,
- Brant C. Faircloth ORCID: orcid.org/0000-0002-1943-0217 17 ,
- Julia Haag ORCID: orcid.org/0000-0002-7493-3917 10 ,
- Peter Houde ORCID: orcid.org/0000-0003-4541-5974 18 ,
- Joel Cracraft ORCID: orcid.org/0000-0001-7587-8342 19 ,
- Metin Balaban 20 ,
- Uyen Mai 21 ,
- Guangji Chen ORCID: orcid.org/0000-0002-9441-1155 9 , 22 ,
- Rongsheng Gao 9 , 22 ,
- Chengran Zhou ORCID: orcid.org/0000-0002-9468-5973 9 ,
- Yulong Xie 2 ,
- Zijian Huang 2 ,
- Zhen Cao 23 ,
- Zhi Yan ORCID: orcid.org/0000-0003-2433-5553 23 ,
- Huw A. Ogilvie ORCID: orcid.org/0000-0003-1589-6885 23 ,
- Luay Nakhleh ORCID: orcid.org/0000-0003-3288-6769 23 ,
- Bent Lindow ORCID: orcid.org/0000-0002-1864-4221 24 ,
- Benoit Morel 10 , 11 ,
- Jon Fjeldså ORCID: orcid.org/0000-0003-0790-3600 24 ,
- Peter A. Hosner ORCID: orcid.org/0000-0001-7499-6224 24 , 25 ,
- Rute R. da Fonseca ORCID: orcid.org/0000-0002-2805-4698 25 ,
- Bent Petersen ORCID: orcid.org/0000-0002-2472-8317 7 , 26 ,
- Joseph A. Tobias ORCID: orcid.org/0000-0003-2429-6179 27 ,
- Tamás Székely ORCID: orcid.org/0000-0003-2093-0056 28 , 29 ,
- Jonathan David Kennedy 30 ,
- Andrew Hart Reeve ORCID: orcid.org/0000-0001-5233-6030 24 ,
- Andras Liker 31 , 32 ,
- Martin Stervander ORCID: orcid.org/0000-0002-6139-7828 33 ,
- Agostinho Antunes ORCID: orcid.org/0000-0002-1328-1732 34 , 35 ,
- Dieter Thomas Tietze ORCID: orcid.org/0000-0001-6868-227X 36 ,
- Mads F. Bertelsen 37 ,
- Fumin Lei ORCID: orcid.org/0000-0001-9920-8167 38 , 39 ,
- Carsten Rahbek ORCID: orcid.org/0000-0003-4585-0300 25 , 30 , 40 , 41 ,
- Gary R. Graves ORCID: orcid.org/0000-0003-1406-5246 30 , 42 ,
- Mikkel H. Schierup ORCID: orcid.org/0000-0002-5028-1790 6 ,
- Tandy Warnow 43 ,
- Edward L. Braun ORCID: orcid.org/0000-0003-1643-5212 44 ,
- M. Thomas P. Gilbert ORCID: orcid.org/0000-0002-5805-7195 7 , 45 ,
- Erich D. Jarvis 46 , 47 ,
- Siavash Mirarab ORCID: orcid.org/0000-0001-5410-1518 48 &
- Guojie Zhang ORCID: orcid.org/0000-0001-6860-1521 2 , 4 , 9 , 49
Nature volume 629 , pages 851–860 ( 2024 ) Cite this article
34k Accesses
13 Citations
980 Altmetric
Metrics details
- Evolutionary biology
- Genome evolution
- Molecular evolution
- Phylogenetics
Despite tremendous efforts in the past decades, relationships among main avian lineages remain heavily debated without a clear resolution. Discrepancies have been attributed to diversity of species sampled, phylogenetic method and the choice of genomic regions 1 , 2 , 3 . Here we address these issues by analysing the genomes of 363 bird species 4 (218 taxonomic families, 92% of total). Using intergenic regions and coalescent methods, we present a well-supported tree but also a marked degree of discordance. The tree confirms that Neoaves experienced rapid radiation at or near the Cretaceous–Palaeogene boundary. Sufficient loci rather than extensive taxon sampling were more effective in resolving difficult nodes. Remaining recalcitrant nodes involve species that are a challenge to model due to either extreme DNA composition, variable substitution rates, incomplete lineage sorting or complex evolutionary events such as ancient hybridization. Assessment of the effects of different genomic partitions showed high heterogeneity across the genome. We discovered sharp increases in effective population size, substitution rates and relative brain size following the Cretaceous–Palaeogene extinction event, supporting the hypothesis that emerging ecological opportunities catalysed the diversification of modern birds. The resulting phylogenetic estimate offers fresh insights into the rapid radiation of modern birds and provides a taxon-rich backbone tree for future comparative studies.
Similar content being viewed by others
Dense sampling of bird diversity increases power of comparative genomics

Evolutionary dynamics of genome size and content during the adaptive radiation of Heliconiini butterflies
American mastodon mitochondrial genomes suggest multiple dispersal events in response to Pleistocene climate oscillations
Understanding the evolutionary relationships among species is fundamental to biology, not only as an account of speciation events but also as the basis for comparative analyses of trait evolution. However, for deep phylogenetic relationships, different studies often show incongruence across analyses 5 , 6 . Large amounts of data may be required to resolve certain relationships yet others can remain recalcitrant even with genome-scale efforts, particularly for rapid radiations 7 , 8 . Phylogenomic incongruence can point to statistical and systematic errors but is also increasingly linked to complex biological processes that accompany rapid diversification 9 , 10 . Prime examples of this problem are the phylogenetic relationships among modern birds (Neornithes), which are inconsistently resolved even with large-scale datasets 1 , 2 , 3 , 11 . The widespread incongruences in evolutionary histories across avian genomes 1 , 12 , 13 has left the phylogenetic relationships of major extant groups unclear and possibly irresolvable 14 .
Modern birds comprise three major groups: ratites and tinamous (Palaeognathae), landfowl and waterfowl (Galloanseres) and all other living birds (Neoaves). The early Neoaves experienced rapid diversification into at least ten major clades 15 , the so-called ‘magnificent seven’ and three ‘orphans’ 12 , encompassing 95% of extant species and a significant portion of their phylogenetic diversity. Due to the short internal branches between these clades, their relationships remain contentious 1 , 2 , 3 , 16 . Furthermore, the timing of the radiation of these major groups is debated 17 , 18 . The ‘mass survival’ scenario places the radiation before the Cretaceous–Palaeogene (K–Pg) mass extinction (66.043 ± 0.011 million years ago (Ma) 19 ), requiring survival of multiple neoavian lineages through the global changes caused by the Chicxulub impact 11 , 17 , 20 . The alternative ‘big bang’ scenario implies a rapid diversification of neoavian groups following the mass extinction, driven by adaptive radiation into new habitats and in the absence of competitors 21 . Fossil evidence supports the scenario of morphological diversification following the K–Pg event 22 . Several molecular studies also supported rapid divergences 1 , 2 , 3 , yet wide credible intervals allowed for the possibility that some of the earliest neoavian divergences predated the K–Pg boundary 23 . Uncertain placement of key taxa and a wide range of time estimates also persist within Passeriformes, the largest avian order with over 6,000 living species 3 , 24 .
Efforts to resolve high-level avian phylogeny face two major challenges. First, it is difficult to obtain large numbers of orthologous loci with suitable properties for phylogenetic analyses. Many studies have been limited to conserved genomic regions such as protein-coding sequence (exons) and ultraconserved elements (UCEs) 2 , 25 . Conserved regions exhibit complex patterns of sequence evolution: for example, selection to maintain protein structure and function places constraints on exon evolution 12 . Standard models of sequence evolution practical for large datasets exhibit poor fit to these regions, and model misspecifications probably result in topological discrepancies across data types 1 , 12 , 13 . Analysis of large numbers of loci does not remove, but can instead reinforce, biases introduced by model violations 1 , 7 . In principle, data types under lower selective pressure such as introns and intergenic regions are preferable; intergenic regions are arguably ideal because they are less probably under strong selection 13 . The second challenge is collecting genomic data from sufficient numbers of species, given that dense taxon sampling can improve phylogenetic estimation 26 , 27 . Thus, the debate in avian phylogenetics has revolved around the trade-off between using diverse loci extracted from entire genomes but for few species (one genome per taxonomic order) 1 or using a smaller number of potentially biased loci sampled from more species 2 , 3 . Both approaches have shortcomings. The most compelling solution is also the most challenging: to create comprehensive datasets with whole genomes sampled across many taxa that inform on deeper timescales.
Here, as one of the main missions of the ‘family phase’ of the Bird 10K Genomes Project (B10K) 28 , we generated a phylogeny for modern birds by sampling across genome assemblies of 363 species representing 218 families (92% of the total) 4 ( Supplementary Data ). We analysed nearly 100 billion nucleotides (around 275 Mb for each species; Extended Data Fig. 1a ), an alignment 50 times the size of the largest available dataset of 48 species 1 (Extended Data Fig. 1b ). As our main data source, we used evenly spaced sampling of intergenic regions across 10 kb windows of a whole-genome alignment 4 (Extended Data Fig. 1c ). We found that selection of a 1 kb locus within the first 2 kb of each window balanced phylogenetic informativeness against the inclusion of recombination within loci (Extended Data Fig. 1d and Methods ). This resulted in 94,402 loci of 1 kb from which we removed those that overlapped with exon and intron regions, resulting in a set of 63,430 purely intergenic loci (in total, 63.43 megabase pairs). In addition to analysis of this main set we tested the effect of various factors, including additional introns and exons, describe the major sources of phylogenetic incongruence and identify the remaining cases of uncertainty.
Intergenic regions resolve deep branches
Our main phylogenetic tree (called ‘main tree’) was obtained by analysis of the 63,430 intergenic loci within a coalescent-based framework (Fig. 1 and Extended Data Figs. 2 and 3 ). We focus on this tree because the findings reported below show that intergenic regions reduce systematic error due to model misspecifications—results that match a priori expectations and previous analyses 12 , 29 . The use of a coalescent-based method 30 , 31 accounts for well-documented incomplete lineage sorting (ILS) in early Neoaves 1 , 32 . A concatenated analysis of the same 63,430 loci (Extended Data Fig. 4 ) resulted in a similar tree that differed in only ten of the 360 branches (2.8%). In these topologies, 98.1% of nodes had full statistical support (main tree, three nodes below 1.00 posterior probability; concatenation, seven nodes below 100% bootstrap support). Although our main topology differed from that of all previous studies, it was more similar to the genome-wide ‘TENT’ tree from ref. 1 of 48 species than to the main topology from ref. 2 , which was based mostly on protein-coding genes of 198 species (Extended Data Fig. 5 ).
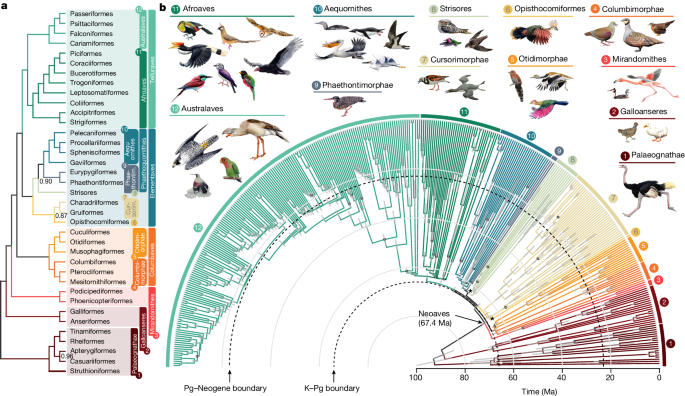
a , Topology simplified to orders with higher clade names following ref. 50 . Numbers on branches represent local posterior probability if below 1. b , Time tree of all species. Grey bars represent 95% credible intervals for age estimation; dots indicate nodes with fossil calibrations; asterisks mark the three branches lacking full support. A tree with tip labels is shown in Extended Data Figs. 2 and 3 .
Within Neoaves we resolve four major clades (Fig. 1a ), three of which are Mirandornithes (grebes and flamingos), Columbaves (Columbimorphae (doves, sandgrouse and mesites) and Otidimorphae (cuckoos, bustards and turacos)), in addition to Telluraves (higher landbirds including Afroaves and Australaves). The fourth major clade is new and phenotypically diverse, containing Aequornithes (pelicans, tubenoses, penguins and loons), Phaethontimorphae (kagu, sunbittern and tropicbirds), Strisores (nightbirds, swifts and hummingbirds), Opisthocomiformes (hoatzin) and Cursorimorphae (shorebirds and cranes). This clade was supported in coalescent-based analyses of intergenic regions and UCEs, but not by exons, introns or in concatenated analysis of intergenic regions (Fig. 3d and Extended Data Fig. 4 ). We name this clade Elementaves because its lineages have diversified into terrestrial, aquatic and aerial niches, corresponding to the classical elements of earth, water and air, and several Phaethontimorphae have names derived from the sun, representing fire.
Most Neoaves diversified post K–Pg
To time calibrate our main tree we empirically generated calibration densities for 34 nodes using 187 fossil occurrences ( Supplementary Information ) and applied these in a Bayesian sequential-subtree framework ( Methods ). We estimated branch lengths from intergenic regions and excluded loci that had evolved at the lowest and highest rates, and also those with the greatest rate variation across lineages. Our analysis produced age estimates with 95% credible intervals that were considerably narrower than previously achieved (Extended Data Fig. 6a ). The widest credible intervals were observed for nodes positioned furthest from the calibration points, including the secondary calibrations involved in subtree dating. The prospects for narrowing these intervals are promising, through future refinement and the addition of fossil-based age constraints. In contrast to a recent study proposing a diversification of Neoaves during the Upper Cretaceous 11 , we found that the early divergences in Neoaves were tightly associated with the K–Pg boundary (Fig. 1b ). Only two divergences occurred before the boundary: Mirandornithes diverged from the remaining Neoaves 67.4 Ma (95% credible interval 66.2–68.9) and Columbaves diverged 66.5 Ma (95% credible interval 65.2–67.9). All subsequent divergences postdate the boundary, although the 95% credible interval of the divergence time between Telluraves and Elementaves and the crown age of Elementaves spans the K–Pg boundary. This evolutionary timeline, wherein only a few neoavian lineages diverged before the K–Pg event, is reflected in all alternative dating analyses ( Methods and Extended Data Fig. 6b–e ), highlighting the robustness of our estimated chronology. This lends more support to a post-K–Pg diversification of Neoaves than previous studies, where the 95% credible interval of between ten and 18 of the nodes allowed for pre-K–Pg divergences 1 , 2 , 18 , 23 .
Abundant discordance among gene trees
Assessing the level of incongruence between gene trees (GTs) across the tree, order-level relationships ranged from showing little or no discordance to high levels of discordance (measured by the quartet score; Fig. 2a ). The percentage of GT quartets matching a species-tree branch at the ordinal level ranged from 99.9 to 33.7% (close to one in three, which corresponds to a polytomy). In particular, 14 nodes had quartet support below 37%. These are the same nodes that have proved difficult to resolve in past studies 15 . For 29 out of 33 nodes, the quartet support of the main topology was significantly higher than both alternatives (one-sided χ 2 test with Benjamini–Hochberg multiple test correction), consistent with expectations under ILS models. We discuss the remaining nodes (26, 39, 43 and 49 in Fig. 2a ) below.
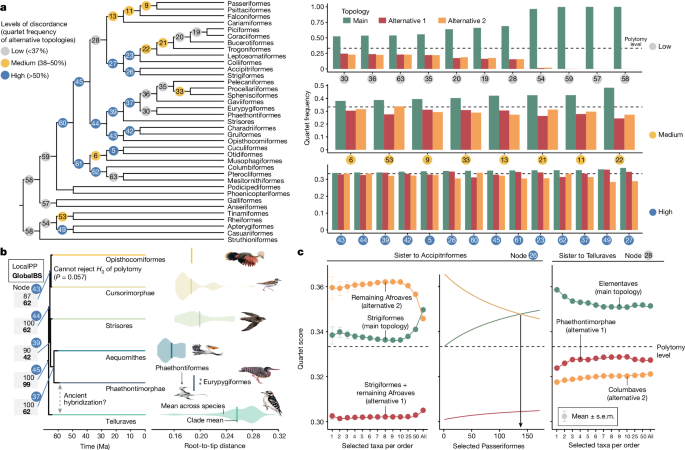
a , Gene tree discordance across the backbone of the main tree. Node colours and numbers represent the bar plots of quartet frequencies for three possible resolutions around each branch. b , Uncertainty at the base of Elementaves. Phaethontimorphae + Aequornithes had high local posterior probability (LocalPP), but global bootstrap resampling (GlobalBS) showed support for an alternative placement. Violin plots (points for the species-poor Phaethontiformes) show higher root–tip distances of Phaethontiformes, and particularly for Eurypygiformes, than Aequornithes, which may cause attraction to the long-branched Telluraves. Further, the placement of Opisthocomiformes is the only branch where a null hypothesis ( H 0 ) of a polytomy cannot be refuted. c , Addition of taxa occasionally affects topology and support. Across 41,918 GTs with at least one species from each group, the alternative placement of Afroaves + Accipitriformes had higher quartet support when only a few species were sampled but declined with increasing taxon sampling (left), particularly of Passeriformes. The main topology dominated when 138 or more passerines were sampled (middle, arrow). Support for Telluraves + Elementaves decreased with increasing taxon sampling (right).
Source Data
Mirandornithes is sister to other Neoaves
The placement of Mirandornithes (also called Phoenicopterimorphae 33 ) as the sister lineage to the remaining Neoaves was supported by both the main tree and concatenation. Although this topology was reported previously 3 it differs from the TENT tree from ref. 1 , which grouped Mirandornithes and Columbimorphae into a clade called Columbea. In our main tree, Columbimorphae combined with Otidimorphae to form Columbaves. This clade has also been reported previously, albeit with low bootstrap support 2 . Mirarab et al. 34 showed that a 21 Mb outlier region of chromosome 4 with abnormally strong signal for Columbea (potentially due to the effects of ancient interchromosomal rearrangements) is responsible for the previous recovery of Columbea. However, with additional taxon sampling of Otidimorphae and Columbimorphae, the effect of this outlier region gradually lessened in favour of an increasingly dominant signal from the rest of the genome that placed Mirandornithes as the sister to other Neoaves (Extended Data Fig. 7a ). In the concatenated analysis, Mirandornithes and Columbimorphae are successive sister groups to the remaining neoavian clades but with limited support (bootstrap = 64; Extended Data Fig. 4 ). Finally, when analysing exons, Mirandornithes were placed deeper in Neoaves as sister to Aequornithes + Phaethontiformes (Extended Data Fig. 4 ), which may relate to previous association with mostly aquatic birds in studies analysing large portions of coding regions (sister to Charadriiformes 2 , Opisthocomiformes + Aequornithes + Phaethontimorphae 11 ).
There is a rapid succession of nodes in this part of the tree, with only 0.92 Ma between the divergence of Mirandornithes and of Columbaves from other groups. Within Columbaves, Otidimorphae has been found in some studies 1 , 2 but not in others 3 , 12 . Within Otidimorphae we resolved Otidiformes as the sister group to Cuculiformes, like some studies 12 but unlike several others 1 , 2 , 3 . The difficulty in this case could be explained by the very short branch (0.57 Ma) separating Otidiformes and other Otidimorphae. Similarly, Columbiformes diverged from the remaining Columbimorphae within 0.26 Ma. These fast divergences partially explain why previous analyses with fewer data led to conflicting resolutions of these earliest neoavian branches.
Waterbirds are deep in a diverse clade
Unlike previous hypotheses that placed Phaethoquornithes (Aequornithes + Phaethontimorphae) as sister to landbirds 1 , 3 , the main tree placed Phaethoquornithes deep inside the diverse Elementaves (Fig. 1a ). The ‘orphans’ Charadriiformes and Gruiformes were consistently grouped together (forming Cursorimorphae), as found in some other studies 1 , 3 . The placement of the third orphan, Opisthocomiformes, as the sister to this group (with a short branch of 0.58 Ma) was the sole instance across the entire phylogeny with statistically indistinguishable levels of GT support for all three possible configurations around this branch 35 (node 43 in Fig. 2b ), a noteworthy finding given the extensive amount of available data.
Whereas the main tree placed Phaethontimorphae as the sister to Aequornithes, further investigations showed a competing placement as the sister lineage to Telluraves. Both topologies have previously been reported 1 , 2 , 3 , 12 , with their difference attributed to the effects of using protein-coding (Phaethontimorphae + Aequornithes) versus non-coding regions (Phaethontimorphae + Telluraves) 15 . We found instead that both topologies have support in the intergenic data. Whereas Phaethontimorphae + Aequornithes had a slightly better quartet score, it was recovered in only 60% of trees resulting from random subsampling of half of the 63,430 loci (Extended Data Fig. 7b ). The two alternative positions of Phaethontimorphae, which are three branches (9.1 Ma) away, each had full local support (posterior probability = 1.0). Nevertheless, global bootstrap support estimated from resampling of GTs showed uncertainty in the three nodes connecting the two placements (global bootstrap = 42–62; Fig. 2b ). Two hypotheses could explain this non-local uncertainty, the first being ancient hybridization between ancestral Phaethontimorphae and Telluraves 3.96 Ma after their divergence. Alternatively, the high support for the alternative placement could be due to problems arising from long branches. Phaethontimorphae have around 25% longer terminal branches than Aequornithes (paired t -test across loci, P < 2.2 × 10 −16 ), showing greater similarity to Telluraves in this regard (Fig. 2b ). Consistent with this explanation, topological changes resulted from data filtering that targeted long branches (clocklikeness, stemminess, total coverage and tree length; Extended Data Fig. 7c ).
Our main tree placed Strisores (also called Caprimulgiformes 33 ) with Phaethoquornithes with moderate support (posterior probability = 0.90; Fig. 1a ), but the concatenated tree grouped them as sister to Telluraves with low support (bootstrap = 32; Extended Data Fig. 4 ). Quartet frequencies did not follow an ILS-alone scenario, because moving Strisores to the base of Elementaves had quartet frequencies similar to the main tree ( χ 2 test, P Benjamini–Hochberg adjusted = 0.317, node 39), but the third alternative had lower frequency ( P = 0.488 × 10 −11 ). Possible explanations include hybridization or long branch attraction, because Strisores have 4–28% longer branches than the other Elementaves, which may attract them to the long-branched Telluraves (Fig. 2b ). Previous studies also failed to find unequivocal support for the relationship of Strisores, placing it as sister to Otidimorphae 1 , Cursorimorphae 11 , Opisthocomiformes 3 or all other Neoaves 2 . Within Strisores our tree positioned Caprimulgidae (nightjars), rather than Sedentaves (oilbird + potoos) 12 , as sister to all others (Extended Data Fig. 2 ), as found previously 2 , 11 .
Difficult placement of owls and hawks
Within Telluraves our main tree supported the proposed split into Australaves and Afroaves 1 , 3 in contrast to other studies 2 , 11 . Our tree grouped Accipitriformes and Strigiformes as the sister to the remaining Afroaves, similar to previous coalescent-based analyses 1 . Concatenated analyses 1 , 3 , including ours, supported Accipitriformes alone as sister to the remaining Afroaves (Extended Data Fig. 4 ). This node also showed quartet frequencies that were statistically indistinguishable for two topologies (35 versus 34.6%, χ 2 test, P Benjamini–Hochberg adjusted = 0.130), but the third was significantly lower (30.5%, P < 10 −16 ; node 26 in Fig. 2a ), contradicting expectations of ILS. Because we found no evidence of long branch attraction (Extended Data Fig. 7d ), the non-ILS patterns could be indicative of ancestral hybridization 36 . In contrast to GTs, direct analysis of alignment sites using CoalHMM ( Methods ) supported an ILS-like pattern in which the two alternative topologies had similar scores (31.2 versus 29.6%). However, CoalHMM assumes ILS a priori and only a strong signal of hybridization can lead to inferring unbalanced quartet frequencies. Thus an ancestral hybridization event, albeit too weak to be detected by CoalHMM, remains plausible. In addition, we observed that the relationship between Accipitriformes and Strigiformes depended on the number of passeriform taxa sampled. The main topology was obtained only when at least 138 Passeriformes were included, whereas sampling fewer taxa of each order favoured Accipitriformes as the sister to the remaining Afroaves (Fig. 2c ). This case demonstrates that the effect of taxon sampling of one group can extend to others and that these sampling effects are not easily predictable.
Insights into the passerine radiation
Our analyses of phylogenetic relationships among Passeriformes (perching birds) included 173 species in 121 families and seven fossil calibrations. The most recent common ancestor of Passeriformes was dated to 50.7 Ma (95% credible interval 48.3–53.0; Fig. 1 ). This estimate is broadly similar to those from other studies with broad taxon sampling (47–53 Ma (refs. 2 , 3 , 23 , 24 )), whereas a previous genomic study that included only five passeriforms found a considerably younger age (39 Ma (ref. 1 )). The split between Tyranni (Suboscines) and Passeri (Oscines) was estimated at 47.3 Ma (95% credible interval 45.1–49.8; Extended Data Fig. 3 ), in line with a previous study 2 , but 3–4 Ma older than other estimates 3 , 24 . Tyranni and Passeri were estimated to have started diversifying around the same time whereas other studies supported a 3 Ma difference between the onset of their diversification 2 , 3 . The three main clades of Tyranni (Eurylaimides, Tyrannides and Furnariides) were inferred to be 4–12 Ma younger than previously found 37 . In Passeri, the age of Corvides was estimated to 25.7 Ma (95% credible interval 23.8–27.7), agreeing with some previous estimates 24 but over 5 Ma younger than others 3 . The divergence of a major subclade of Passerides (Sylviida + Muscicapida + Passerida) was inferred to have occurred shortly after the Palaeogene–Neogene boundary (22.4 Ma, 95% credible interval 20.6–24.2; Extended Data Fig. 3 ) whereas previous studies placed its divergence before the boundary 3 , 23 , 24 . This branch and some subsequent divergences occurred in close succession, indicating rapid diversification.
In Passeri, our tree differed from studies based on UCEs or 5′-untranslated region sequences 3 , 24 , 38 , including in the positions for Orioloidea, Malaconotoidea, Corvoidea, Mohouidae, Neosittidae, Regulidae and Urocynchramidae (Fig. 3d (asterisks) and Supplementary Information ). Some of these difficulties also appear to be related to rapid diversification, seen for example in the extremely short internode of Mohouidae (0.18 Ma).
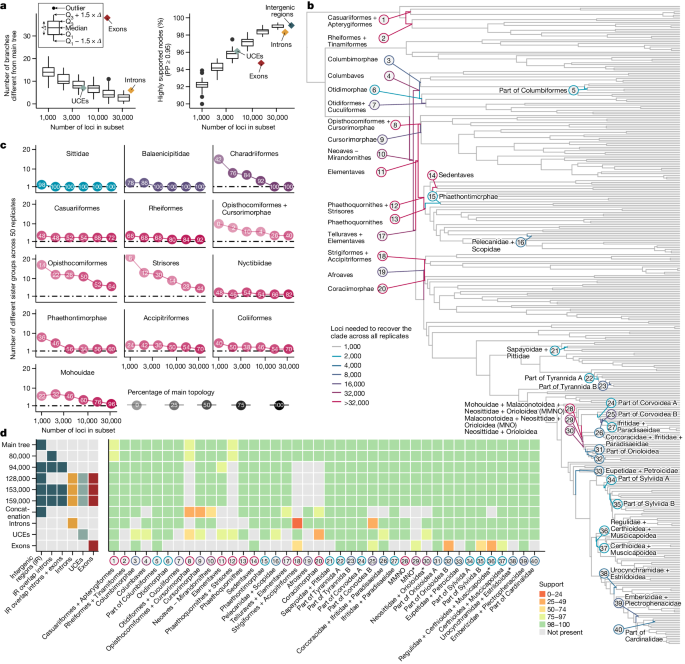
a – c , Species trees were reconstructed from subsets of GTs (1,000, 2,000, ..., 32,000) of the 63,430 intergenic regions in 50 replicates. a , The addition of loci increases similarity to the main tree (left) and increases the proportion of highly supported nodes (right). b , The main tree, with branches coloured according to the difficulty involved in consistently recovering the clade across subsets. Most branches were consistently obtained with only 1,000 GTs (grey); the remaining 40 branches required more loci. c , Increasing the number of loci decreases the number of possible sister groups. We recorded the number of unique sister groups for each node across subsets. Colours correspond to the difficulty (from b ), and shading and number show the frequency, with which the main topology was obtained. The top row illustrates examples of easy nodes. in which the same sister group was consistently recovered with 2,000, 4,000 and 16,000 loci, respectively. The remaining plots show the most difficult nodes, in which multiple sister groups were supported even when 32,000 loci were subsampled. d , Ten selected species trees, data types used in each and the support for all challenging branches (labelled in b ). Asterisks indicate relationships in Passeriformes that differ from previous studies. MNO, Malaconotoidea + Neosittidae + Orioloidea; MMNO, Mohouidae + MNO, PP, posterior probability; Q, quartiles.
Rheas have conflicting placements
Outside of Neoaves we found support for different relationships of Rheiformes within Palaeognathae, a conflict previously attributed primarily to ILS 39 . Whereas our main topology found Rheiformes as the sister to Tinamiformes, analysis with CoalHMM put it as sister to Apterygiformes + Casuariiformes (Extended Data Fig. 7g ), in agreement with that previous study 39 . We found that Rheiformes and Tinamiformes had a higher proportion of loci with high guanine–cytosine (GC) content than other taxa (Extended Data Fig. 7e ). We observed that omission of loci with similar GC content for Tinamiformes and Rheiformes, but not for others, tended to reduce (but not eliminate) support for this clade (Extended Data Fig. 7g ). These results suggest that the strong support for this grouping in our main tree was enhanced by biased GC content, leaving other placements of Rheiformes (for example, as sister to Apterygiformes + Casuariiformes, as recovered by CoalHMM) as plausible.
Effect of taxon sampling varies
The question of whether to sample more species or more genetic loci is pivotal in phylogenetic study design 40 . Whereas expansion of taxon sampling helps to mitigate the confounding impact of long branches within GTs 26 , 41 , its effects on species-tree inference are less clear. To investigate this question we randomly selected between one and ten species for each order and constrained the 63,430 intergenic GTs to the selected taxa before rescoring the species tree. These changes in taxon sampling affected ordinal relationships in only three cases (Extended Data Fig. 7f ), with the aforementioned Accipitriformes + Strigiformes being the strongest example (Fig. 2c ). More frequently we observed that increasing taxon sampling affected only the amount of GT discordance but not the topology (for example, Telluraves + Elementaves in Fig. 2c ). Thus our results are relatively robust to taxon sampling, although with some exceptions.
Number of loci needed varies across nodes
As access to large numbers of loci becomes common, the choice of how many and which loci to select is a fundamental decision 42 . Using repeated subsets of the 63,430 dataset, we found that greater locus sampling resulted in trees more similar to the main tree and with higher support (Fig. 3a ). The same trend was observed across all partitions of the genome (intergenic regions, introns, UCEs and exons; Extended Data Fig. 8a,b ) and with other species trees as reference, except the purely exonic one (Extended Data Fig. 8c ).
We assessed how many loci were required to consistently recover each clade of the main tree (Fig. 3b ). We found that most clades (321 of 361, 89%) could be identified with just 1,000 loci. A minority of clades (30 of 361, 8%) needed substantially more, from 2,000 to 32,000 loci, before analyses could consistently support them (Fig. 3c ). In the remaining ten clades (2.8%) increasing the number of loci reduced incongruence but did not consistently recover the main topology across replicates, even with 32,000 loci (Fig. 3c and Extended Data Fig. 9 ). Most of these difficult nodes were associated with short branches after the K–Pg boundary and within Corvides (Fig. 3b ). For example, mousebirds (Coliiformes), placed in agreement with some studies 1 , 2 , 3 in our main tree, had an alternative placement in 30% of subsets of 32,000 loci, consistent with previously reported difficulties 1 , 14 .
Strong effects of different locus types
Species trees built from GTs of different data types were substantially different, especially between protein- and non-coding data, akin to previous findings 1 , 12 , 13 . The species tree built from 14,355 exon loci (excluding the hypervariable third codon position) differed in 38 of 360 branches from the main tree (compared with six or seven differences for the other data types; Extended Data Fig. 4 ). Beyond dissimilarity to the main tree (Fig. 3d ), trees inferred from exons were less internally consistent—they were more sensitive to subsampling than trees built from other data types (Extended Data Fig. 8a–c ). Even when controlling for the number of GTs used in species-tree construction, exons produced more variable trees than other data types (Fig. 4a ).
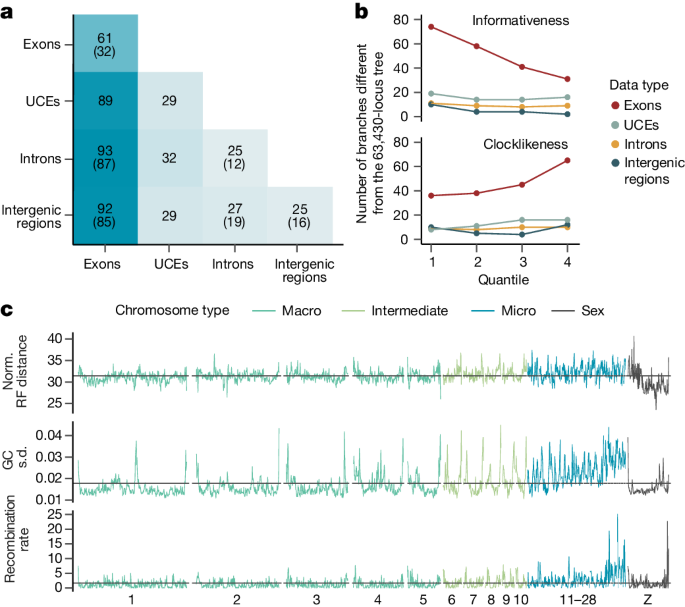
a , Protein-coding regions yield more varied species trees when they are subsampled. Each heatmap cell shows the average Robinson–Foulds distance between 1,250 (diagonal, 1,225) pairs of species trees, each built from 2,000 GTs of different data types. Values in parentheses give the same metrics for 8,000 GTs, omitting UCEs with fewer loci. b , Effect of subsetting loci by data type and different metrics. The y axis represents the number of differences to the main tree; the x axis shows two metrics split into four quartiles, from low to high. Phylogenetic informativeness is the proportion of parsimony-informative sites. Clocklikeness is the coefficient of variation in root–tip distances, a measure of branch length heterogeneity. Extended Data Figure 8g shows other metrics. c , Patterns of phylogenomic incongruence along the genome. Using the 94,402 loci binned approximately every 500 kb, lines show Robinson–Foulds (RF) distances to the main tree (top), variance in GC content (middle) and recombination rate (bottom). Horizontal lines indicate genome-wide averages.
We found that data types differed in regard to the risk of violating assumptions of phylogenetic models. A much higher proportion of exonic loci was found to be at risk of sequence saturation (30.83%) compared with the other data types (intergenic regions, 0.07%; UCEs, 0.34%; introns, 0.83%). The evidence for violation of stationarity was generally low, yet highest among exons (exons, 2.45% of loci failing the test; UCEs, 0.02%; intergenic regions, 0.07%; introns, 0.08%). Moreover, because individual exons of the same gene were combined into one locus, the assumption that phylogenetic loci are recombination free is expected to be more frequently violated by exonic loci. An exonic locus can span wide stretches of the genome because its individual exons are not contiguous (mean sequence length 16,964 base pairs, range 149–566,199) as opposed to loci of other data types (mean sequence length: introns, 2,543 base pairs; UCEs, 2,095 base pairs; intergenic regions, 897 base pairs). Because the increased length of exons increases the risk of within-locus recombination, analysis of only intergenic regions minimizes the risk of recombination and model violations.
We found that exonic loci had less phylogenetic information and were more variable in their signal than the other data types (Extended Data Fig. 8d,e ). Exons also scored highest in a measure of phylogenetic estimation difficulty (Extended Data Fig. 8f ), indicating that their GTs are less reliable than those of other data types. To examine whether exons had a misleading signal, we restricted species-tree inference to GTs with more signal, less gappy alignments, greater clocklikeness and greater total length. Unlike intergenic regions, in which subsampling did not systematically change the species trees, the use of more informative, less gappy and more clocklike exons reduced incongruence between the resulting species trees and the main tree (Fig. 4b and Extended Data Fig. 8g ). Thus exons yield phylogenetic trees that are less reliable. This conclusion is consistent with earlier analyses based on fewer genomes 1 , 12 , 13 , 29 . Our results indicate that the damaging effects of model violation and limited signal of exons are not offset by increased taxon sampling, as one might hope 2 , 43 .
To investigate whether the confounding effects of exons could be swept out by other data, we gradually augmented purely intergenic loci (Extended Data Fig. 1b ). The addition of 1 kb windows overlapping with introns (resulting in a total of 80,047 loci) led to the same topology (Fig. 3d ). However, when windows overlapping with exons were added (94,402 loci), the resulting tree agreed with the main tree on the first four neoavian clades (Mirandornithes, Columbaves, Telluraves and Elementaves) but differed in five difficult branches (Fig. 3d and Extended Data Fig. 4 ). This 94,402-locus topology was also obtained when adding UCEs, purely intronic loci and purely exonic loci (not those overlapping with 1 kb windows) to either the 63,430 set (128,233 loci) or the 94,402 set (159,205 loci). Removal of loci that failed saturation and stationarity tests from the full set (153,789 loci remaining) returned the same tree, albeit with low support on branches conflicting with the main tree. These results indicate that the inclusion of exonic loci, even if these constitute just 10% of the data and are restricted to those that pass the testing of model fit, can affect the most unstable parts of the tree. This finding can partially explain the different topologies reported in other studies using a high proportion of coding regions 2 , 11 . By contrast, exclusion of introns did not make a difference topologically in our analyses. Nevertheless, we treat as uncertain the five branches that differ between purely intergenic regions and these alternative trees (Fig. 3d ).
Discordance along chromosomes
Averaged over 500 kb windows, GT discordance levels were mostly consistent along chromosomes (31.4% normalized Robinson–Foulds distance to the main tree; Fig. 4c ). However, we observed some notable troughs and peaks of GT discordance, particularly around the telomeres and some centromeres (relative to the chicken genome), agreeing with previous findings regarding telomeres 1 . Gene trees inferred from macrochromosomes (below 50 Mb) were slightly less distant to the main tree than intermediate chromosomes (12–40 Mb) and microchromosomes (average size 12 Mb; Extended Data Fig. 10a ). The higher discordance near telomeres and across microchromosomes may be related to their elevated richness of genes, variation in GC content and higher recombination rates (Fig. 4c and Extended Data Fig. 10b–d ) leading to higher local effective population size and challenging phylogenetic reconstruction. The Z chromosome had the lowest discordance (Extended Data Fig. 10a ), consistent with its lower recombination rate. Species trees inferred from individual chromosomes resulted in topologies with 1–3% difference to the main tree, with most differences observed in microchromosomes followed by intermediate chromosomes (Extended Data Fig. 10a ).
Implications for avian diversification
We next evaluated how well the new phylogenetic tree reflects avian morphology, testing the expectation that closely related species should resemble one another. We found that our main tree fits morphological traits better than the topology of ref. 2 , even when controlling for taxon sampling (Fig. 5a ), including the larger number of Passeriformes in our study (supplementary results given in Supplementary Information ). Simulations considering the misplacement of taxa and convergent scenarios suggested that the higher phylogenetic signal in this comparison was more probably attributed to topological differences (Extended Data Fig. 11a ).
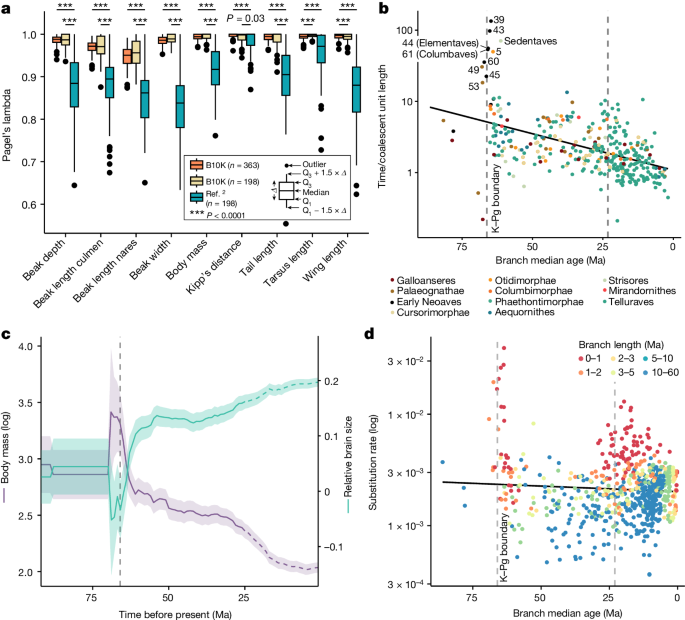
a , The main tree fits morphological traits well. We measured phylogenetic signal (Pagel’s lambda) for nine traits over 100 replicates and compared the fit based on (1) the main tree, (2) the ref. 2 topology and (3) the main tree with random species sampling to match the sample size used in ref. 2 (one-sided t -test with Bonferroni correction). b , The K–Pg and Palaeogene–Neogene transitions were associated with increased effective population sizes of some lineages. Shown are the midpoint ages of each branch compared with the ratio between its length in time units and in coalescent units, which is proportional to the effective population size of that branch and its generation time. Numbers correspond to selected nodes from Fig. 2a . c , Variations in body mass and relative brain size over time changed in different directions following the K–Pg event. Solid lines indicate mean values and ribbons mark 95% confidence intervals. The dashed parts of the reconstruction (from 25 Ma) indicate possible uncertainty due to the lack of within-family sampling (Extended Data Fig. 11g ). d , Substitution rates increased around the K–Pg boundary. Estimated molecular rates for the intergenic regions are plotted against the midpoint age of each branch.
Next we compared branch lengths in time units and coalescent units, which should be proportional to population size, ignoring the effect of varying generation time ( Methods ). We found a strong signal of increased population sizes on nearly half of the branches 0–2 Ma following the K–Pg transition (Fig. 5b ), in agreement with an earlier analysis of insertions and deletions 44 . This pattern could be indicative of lineages undergoing density compensation, a transient increase in population size in response to ecological opportunity and release that may be associated with adaptive radiation 45 . Birds would have been well positioned to exploit landscapes newly devoid of competitors and predators following the K–Pg mass extinction because of their flight capabilities. Vagile insectivores and marine species such as Strisores and Aequornithes could have rapidly expanded into early-succession habitats. A less marked spike was also observed around the end of the Palaeogene (Fig. 5b ). There was also an apparent gradual decline in the ratio of time and coalescent unit branch lengths by close to an order of magnitude over 60 Ma. A reduction in generation times could plausibly produce this result, possibly reflecting an increase in numbers of passerine families through time. There has also been a trend toward reduced inferred body sizes over this time (Fig. 5c ), and it has long been appreciated that taxa with small body size have short generation times 46 .
Substitution rate estimates for the intergenic regions also showed a strong increase at and shortly after the K–Pg boundary (Fig. 5d ), and a more diffuse increase near the boundary to the Neogene. The rate increase near the K–Pg boundary has been noted for other data types and attributed, at least in part, to the ‘Lilliput effect’ (refs. 47 , 48 ). This refers to decreases in body size in the wake of mass extinctions; those changes in body size would affect other life history traits, such as generation time. Consistent with this explanation, we found a decrease in reconstructed body size following the K–Pg event (Fig. 5c ). This was accompanied by an increase in inferred relative brain size shortly before the K–Pg event, suggestive of strong selection for adaptability or behavioural flexibility, consistent with previous findings 49 . Shortly after the K–Pg event, the continuous changes of inferred relative brain size appear to have ceased (Fig. 5c ). From around 35 Ma the reduction in reconstructed body mass does not seem to have been accompanied by an increase in relative brain size.
Across the tree we found that rapid evolutionary change occurred at the origin of major clades, throughout the diversification of some clades and along some isolated branches. Passeriformes exhibited a burst of body mass evolution at their most recent common ancestor (Extended Data Fig. 11b ). Rates of evolution in relative brain size were more variable, with rapid evolutionary change in some clades (for example, Telluraves, vocal learning lineages such as parrots, corvids and hummingbirds) 49 . In addition, our data showed that the early burst was followed by sustained varied rates within these groups, especially in Passeri (Extended Data Fig. 11c ).
Conclusions
Relationships along the backbone of Neoaves have long been contentious, with various analyses yielding incongruent results. At the heart of the disagreements has been a long-standing question: is it better to sample many taxa at a few loci (typically conserved regions, such as exons and UCEs) or sample many loci widely across the genome, even if available from fewer species? We can finally answer this question because our data provide both dense taxon sampling and many loci across the whole genome. We observed that the number of loci, in addition to sequence types (for example, exons, introns, intergenic regions or chromosome type), had a much greater effect on the inferred tree than taxon sampling. Nevertheless, increased taxon sampling was crucial in inferring more precise dates, and for studying traits, trajectories of population size and substitution rates. By focusing on intergenic regions, a source of data largely unused in the past, we minimized model violations and increased phylogenetic resolution. Nonetheless, our results also showed that several recalcitrant relationships remain, even with this wealth of data, due to challenges imposed by biological processes such as hybridization that are hard to model in deep time using phylogenetics. Overall, our results underscore the complexity of genome evolution and show methodologies that are likely to be useful for future phylogenomic studies focused on deep relationships.
Further details on methods are given in Supplementary Information . No statistical methods were used to predetermine sample size. The experiments were not randomized and investigators were not blinded to allocation during experiments and outcome assessment.
Selection of genomic regions for phylogenomic inference
For the main tree, we used putatively intergenic regions extracted from a Cactus whole-genome alignment 4 , 51 . We converted the HAL alignment to MAF format using chicken as the reference and extracted the best-aligned synteny blocks from each query species using 10 kb windows ( https://github.com/Secretloong/Cactus_Alignments_Tools , using HALtools 52 v.2.3), skipping regions that were repetitive in chicken or those present only in Galliformes. Among the first 2 kb of each window, the 1 kb portion with the most site-wise occupancy was selected to avoid portions with few sequences. The decision to use 1 kb loci from which to estimate GTs was made following preliminary assessments (Extended Data Fig. 1d ). Therefore loci were 8–9 kb apart, reducing the risk of strong linkage 53 . We excluded fragmentary sequences (under 50% of the median length of all sequences of the locus) and loci with fewer than four sequences. This resulted in 94,402 loci for which we estimated GTs. Based on the chicken genomic annotation, we identified 1 kb loci which had overlap with exons (14,355 loci) or introns (16,617 loci) and created smaller datasets without these regions (Extended Data Fig. 1b ). Subtraction of these from the total loci resulted in 63,430 purely intergenic loci, which were used to construct the main tree.
We also extracted loci of other data types and applied the filtering described above. This resulted in 44,846 intronic, 14,972 exonic and 4,985 UCE loci. Introns were extracted from the Cactus alignment following previously described procedures 4 , reconstructing individual GTs for each intron of the same gene. Protein-coding regions were obtained from genome annotations 4 and all exons of the same gene were analysed as one locus; these were further filtered and aligned. This was done with an iterative PASTA 54 v.1.8.5 pipeline that included TreeShrink 55 v.1.3.1 to remove outlier sequences, alignment with MAFFT 56 v.7.149b G-INS-i with a variable scoring matrix 57 to isolate potentially unrelated segments and removal of these blocks. We excluded third codon positions because these were previously shown to be problematic 1 . UCE loci were extracted using PHYLUCE 58 v.1.6.3 (commit 185b705) targeting 5,060 UCEs and 1,000 base pair flanking regions. After filtering, 5,006 UCE loci remained. Alignment and exclusion of outliers was conducted similar to the protein-coding regions but using MAFFT L-INS-i without removal of alignment segments.
Generation of GTs and species trees
A total of 159,205 GTs were estimated using maximum likelihood tree inference with Pargenes 59 v.1.1.0, which uses substitution model selection through Modeltest-NG 60 v.0.1.3 and RAXML-NG 61 v.0.9.0, with ten random and ten parsimony starting trees and scaled branch lengths. To identify and collapse poorly supported branches before running ASTRAL we used IQTREE 62 v.1.6.12 to perform parametric approximate likelihood ratio tests (aLRT), which are rapid tests of the three possible nearest-neighbour resolutions around a branch 63 and are more computationally efficient than bootstrapping. Outputs from Pargenes were used for computing aLRT scores. Poorly supported branches were contracted to polytomies using newick-utilities 64 v.1.6 if their aLRT value was below 0.95.
Collapsed GTs were summarized into a coalescent-based species tree using ASTRAL-MP 65 v.5.14.5. Support was assessed using posterior probability. We also performed gene-only, multilocus bootstrapping (globalBS) for cases in which uncertainty is not local (for example, two placements many branches away both resulting in high quartet support), a scenario that can mislead local posterior probability support 66 . In addition we tested polytomy null hypotheses 35 and evaluated the quartet score of the three alternative nearest-neighbour interchanges around each branch 66 . Quartet scores were visualized using DiscoVista 67 . We evaluated alternative species trees (for example, moving Phaethontimorphae) by scoring these trees against the same input GTs using ASTRAL.
For a concatenated analysis of the 63,430 loci under maximum likelihood we used RAXML-NG v.1.0.1, partitioning by locus (63,430 partitions) with their previously determined substitution models. We ran 20 independent searches from random starting trees and picked the highest-scoring tree. We then ran 50 tree searches on bootstrapping pseudo-replicate alignments, judged sufficient according to the extended majority rules (MRE) bootstrap convergence criterion 68 . To save time and energy we used a topological constraint for all tree searches (maximum likelihood and bootstrapping). This was a strict consensus of the ASTRAL trees (63,430 loci, exons, introns and UCEs) and of an initial maximum likelihood run on the 63,430 loci (based on ten tree searches with five random plus five parsimony starting trees, no bootstrapping). This consensus left the backbone nodes free to be inferred with constraining uncontroversial nodes within orders (317 nodes resolved, 45 collapsed).
Fossil calibration and molecular dating
We performed molecular dating using a Bayesian sequential-subtree approach 69 . This involved using date estimates from an initial analysis of a backbone tree (56 tips) containing two representatives of each of 11 subtrees. This provided secondary calibrations for subsequent dating analyses of 11 subtrees (19–42 tips each). The subtrees were then attached to the backbone to assemble a timetree of all 363 taxa.
We performed molecular dating using a subset of the 63,430 loci. For all loci we estimated phylograms in IQTREE 70 v.2.0.4 under GTR + F + R4, fixed to the main topology and rooted with FastRoot 71 . We selected 10,494 loci with the lowest coefficient of variation in root–tip distances, thereby retaining the most clocklike loci. For locus partitioning we randomly divided loci into two groups of 5,247 within which we partitioned based on their macro-, intermediate and microchromosomal origin. The two locus groups were used for dating. Half of the loci were used to date the backbone tree and the other half to date the subtrees, thus avoiding data duplication in the likelihood.
For node-based calibrations we identified 34 clades with fossils fulfilling best-practice criteria 72 ( Supplementary Information ). We used CladeDate 73 to generate calibration densities empirically based on fossil occurrences (187 fossils) and estimators of distributions in which the truncation was the estimated age of the clade 23 , 74 . We used the Strauss and Sadler 75 estimator for uniformly distributed fossil occurrences; otherwise, we excluded the Quaternary record or used estimators that do not assume sample uniformity 73 . The resultant distributions of clade ages were used to fit Student-skew distributions to parameterize calibration priors.
The posterior distributions of the ages of the 11 nodes in the backbone tree that corresponded to the root nodes of the subtrees were fitted with skew- t densities using the R function sn::st.mple v.2.0.0, under the BFGS method for parameter optimization 76 . The skew- t parameters were then used to specify the prior distributions of root ages for dating analyses of the subtrees.
Bayesian molecular dating was conducted using MCMCtree 77 v.4.9h, with approximate likelihood calculation 78 and under the GTR + G model. The analyses included all calibration priors, a minimum bound on root age based on an uncontroversial neornithine fossil 79 and a soft maximum bound at 86.5 Ma. Nodes without calibrations followed a birth–death process prior 80 ( λ = μ = 1, sampling fraction ρ = 0.1), which gives an approximately uniform kernel. We used a relaxed clock with lognormally distributed rates across branches and a gamma-Dirichlet prior on rates across the three subsets of loci 81 . During Markov chain Monte Carlo sampling, samples were drawn every 2,500 steps over a total of 5.5 × 10 7 steps following 5 × 10 6 burn-in, run twice.
We performed four additional analyses with alternative settings (Extended Data Fig. 6 ): (1) uniform calibration priors with ranges spanning the 95% probability density of the original calibration prior, adding a soft maximum bound with a 2.5% tail of probability; (2) a Jurassic age bound with a relaxed maximum age bound of 201.3 Ma on the root; (3) a calibration subset of 23 calibrations that were considered to be the most reliable ( Supplementary Information ); and (4) a set of 10,494 loci randomly selected from the 63,430 set, split into two equal groups of 5,247 and randomly partitioned into three subsets of 1,749 loci.
Subsetting analyses
Taxon sampling.
To investigate the effect of sampling multiple species across orders (which represent the most contentious branches), we successively reduced taxon sampling to 50, 25, 10, … 2 or 1 species per order. We randomly selected species from the existing GTs of the 63,430 locus set, retaining all if fewer than the desired number were available. We then scored the main tree against the taxon-reduced GTs to compute the normalized quartet support for the three topologies around each branch. These analyses showed substantial impact only for Accipitriformes, in which fewer than 50 species were required to recover the main relationship. Because only Passeriformes had fewer than 50 taxa, we inferred that their sampling affected the position of Accipitriformes. To test this we removed 1, 3, ... 171 of the 173 Passeriformes in random order and computed quartet scores with GTs restricted to that subset. Two replicates produced indistinguishable results.
Data quantity
Of the 63,430 loci included in the main analysis we randomly selected subsets of increasing numbers of GT up to maximally half of the available GTs (1,000, 2,000, … 32,000). Each subset was repeated 50 times and an ASTRAL tree was estimated for each. The subset topology was compared to the main tree by counting the number of differing branches (Robinson–Foulds distance/2) using TreeCmp 82 v.2.0 and calculating the proportion of highly supported branches (posterior probability ≥ 0.95). We recorded whether each clade of the main tree was present in subset trees and counted how many different sister groups were present across the 50 replicates of each subset. We performed the same analyses for the other data types, maximally sampling about half of the available loci. This included exons (50 times sampling 1,000, 2,000, … 8,000 GTs), introns (1,000, 2,000, … 32,000) and UCEs (1,000, 2,000). We also performed the analyses using all non-coding (80,047 windows, introns and UCEs totalling 129,878 loci) GTs (1,000, 2,000, … 64,000).
We compared topological differences between trees for each data type, also controlling for the number of GTs used. We subsampled loci at random (50 times). The highest number of GT subsets present across all data types was 2,000 (limited by the number of UCEs). To show the effect of increasing loci we also performed the analysis for 8,000 loci, omitting comparisons with UCEs. We calculated mean pairwise Robinson–Foulds distances between resulting species trees.
Genomic characteristics
For GTs we calculated taxa number, tree length, tree diameter, stemminess, clocklikeness, mean branch support and proportion of branches with aLRT above 95 and above 99. For gene alignments we calculated locus length, total coverage, number and proportion of parsimony-informative sites and mean and s.d. of GC content (with seqkit 83 v.2.2.0). We predicted the difficulty of phylogenetic estimation under maximum likelihood using Pythia 84 v.1.0.0, which estimates whether the alignment is likely to result in multiple, topologically highly distinct yet statistically indistinguishable topologies. We divided loci into four equal-sized quantiles based on their values for each metric (20,011 loci based on 80,047 loci). We then estimated an ASTRAL tree for each quantile and calculated Robinson–Foulds distances to the main tree.
Analysis by chromosomes and chromosomal category
We built 16 species trees from GTs of the 80,047 loci according to their chromosomal assignment in chicken, excluding small chromosomes (fewer than 1,000 GTs, chr15, chr16, upwards from chr21). We also built species trees for each of the chromosome size categories of birds 85 —that is, macrochromosomes (49,686 GTs), intermediate chromosomes (11,592), microchromosomes (12,740) and the Z chromosome (5,672). To investigate discordance within and across chromosomes we calculated Robinson–Foulds distances to the main tree for each of the collapsed GTs from the 94,402 set, normalized to the numbers of nodes in each GT. We investigated potential genomic colocalization with the s.d. of GC content, because high deviations violate common model assumptions, and with recombination rates estimated for chicken 86 . We estimated mean values using the same bins as that study 85 (approximately 500 kb).
Phylogenetic model adequacy
We tested for excessive amounts of non-stationary base composition using Foster’s posterior predictive simulations method 87 , adapted to maximum likelihood using a parametric bootstrap 88 . We also tested for misleading inferences due to substitution saturation using entropy tests on parsimony-informative sites 89 . For both tests, loci were defined as having a high risk of misleading inferences under scenarios in which all simulations yielded inaccurate inferences. We built an ASTRAL tree based on all loci that passed both tests (153,789 loci remaining).
Investigation of specific nodes
CoalHMM was used to estimate ILS levels of two clades that were difficult to resolve in our main analyses, Rheiformes and Strigiformes + Accipitriformes. We filtered and split alignment blocks into 1 Mb chunks on which CoalHMM was run 90 . We tested potential placements of Rheiformes within Palaeognathae using one representative for each order (using the most contiguous genome) and for all chromosomes. CoalHMM was also run for potential placements of Strigiformes and Accipitriformes, using Passeriformes as the outgroup and Bucerotiformes to represent the remaining Afroaves. The best-fitting topology was chosen based on posterior probabilities. Under an ILS model and in the absence of phenomena such as ancient hybridization, the proportion of sites supporting topologies different from the species tree should be equal.
GC content within Palaeognathae
Because we suspected that convergent GC content between Tinamiformes and Rheiformes may affect GT estimation, we defined a measure of GC similarity (∆GC; Supplementary Information ). This should be zero under the stationary models of evolution used for phylogenetic inference. Positive values deviate from the model uniting Tinamiformes + Rheiformes and negative values have the reverse effect. For 54,651 of the 63,430 loci that had all relevant species present, we calculated ∆GC and created nine subsets of loci. We ran ASTRAL on each subset, and all of them united Tinamiformes + Rheiformes. We computed a normalized quartet score around the branch to investigate whether subsets without high ∆GC had lower quartet support for Tinamiformes + Rheiformes.
Inference of effective population size
We compared the time tree with the coalescent unit lengths estimated by ASTRAL. For each internal branch we computed the ratio of the branch length in time units to coalescent unit length:
Higher values are indicative of higher population size ( N e ) or longer generation time. Ignoring changes to generation time, higher time to coalescent unit ratios can be attributed to larger N e . Around the K–Pg boundary the generation times are presumed to have decreased, which makes the increases in our measured quantity indicative of even larger N e growth than what would be inferred if generation times were assumed constant. Note that summary methods such as ASTRAL are known to underestimate coalescent unit length in the presence of high GT estimation error. However, we compare branches only to each other without claiming to estimate the true N e . Thus, estimation error, if it is not particularly concentrated on specific nodes, should not affect the relative values.
Analysis of molecular evolutionary rates
Genome-wide evolutionary rates were estimated for each branch using the 63,430 loci. To minimize the estimation bias in substitution rates arising from discordance between the species tree and GTs 91 , we considered only those GT branches that were concordant with the main tree 92 . Each concordant branch length was divided by the time duration of the branch from the main time tree analysis, leading to a rate estimate for each species-tree branch for each locus.
Analysis of phylogenetic signal
Pagel’s lambda ( λ ) 93 was measured for nine continuous morphological traits from AVONET 94 on the main tree, the topology in ref. 2 and the main tree randomly subsampled to the sample size used in ref. 2 ( n = 198). We also performed a comparison between trees pruned to the 124 families present in both studies. To account for the high proportion of Passeriformes in our study we also excluded all but one passerine from both trees. We calculated λ for each trait using 100 simulations using phylolm 95 . To investigate the potential effects of an incorrect tree topology we simulated traits on the main tree under a Brownian motion model using fastBM 96 with λ = 0.96. We then randomly changed the position of 1, 5, 10 and 20% of taxa to represent incorrect relationships, repeated each 100 times, and estimated λ . To investigate the effect of convergent evolution we randomly selected species pairs consisting of one passeriform and one non-passeriform, representing 1, 5, 10 and 20% of taxa. We gave each species pair the same trait value, repeated 100 times, and estimated λ .
Analysis of body mass and brain size evolution
We obtained body mass data (log-transformed) for 363 species 94 , 97 and estimated brain size (volume of the brain case) for 228 species based on endocast volume, or back-calculated it using brain volume = brain mass/1.036 (ref. 98 ). We used the average of males and females or mean unsexed values when available. For brain size we used missForest 99 to impute missing values based on phylogenetic relationships. Relative brain size was calculated as the residual from a log–log phylogenetic generalized least-square regression of absolute brain size against body mass. Ancestral states of both traits were reconstructed by Evomap using a multiple-variance Brownian motion approach 100 . Variations were summarized by dividing the phylogeny into bins of 1 Ma and averaging in each over all branches.
The rates of evolution in both traits were analysed using BayesTraits 101 v.4 with variable-rates models and default priors. Each analysis ran for 110 million iterations with a burn-in of 10 million in triplicates. We used the convergence diagnostic test of coda 102 and selected the run with the highest mean marginal likelihood. We also compared the fit of three single-process models (Brownian motion, early burst and Ornstein–Uhlenbeck) using Geiger 103 v.2. To compare model fit using Akaike information criterion (AIC) (Extended Data Fig. 11e ), we used the mean of the rate-scaled trees of BayesTraits and calculated the likelihood of a Brownian motion model on this tree with the same trait data 104 . To investigate whether sampling one species per family could affect ancestral reconstructions, we modified tip values to reflect the family’s range in body size 94 across 100 replicates (Extended Data Fig. 11f ). We also confirmed that inclusion of the imputed brain size values did not change the shape of ancestral reconstructions (Extended Data Fig. 11g ).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The genome assemblies analysed in this study and their whole-genome alignment were part of a previous study 4 , and accession numbers are given as part of the Supplementary Data . Alignments, GTs and species trees, in addition to data files produced for their analysis and scripts to plot the figures, are available at https://doi.org/10.17894/ucph.85624f66-c8e5-4b89-8e8a-fe984ca89e4a (ref. 105 ). This repository also contains a file detailing contents and commands to use for individual and batch download of files. The study analysed morphological trait data from AVONET 94 ( https://figshare.com/s/b990722d72a26b5bead ) 106 and Dryad ( https://doi.org/10.5061/dryad.fbg79cnw7 ) 97 , recombination rates for chicken 86 and time-calibrated species trees from ref. 1 ( http://gigadb.org/dataset/101041 ) 107 and ref. 2 (Avian-TimeTree.tre from https://zenodo.org/records/28343 ) 108 . Source data are provided with this paper.
Code availability
Code used for producing the figures in this paper is available at https://doi.org/10.17894/ucph.85624f66-c8e5-4b89-8e8a-fe984ca89e4a (ref. 105 ). The pipeline for extraction of synteny blocks from the whole-genome alignment is available under https://github.com/Secretloong/Cactus_Alignments_Tools . The pipeline for filtering and alignment of loci is available under https://github.com/uym2/TreeShrink/tree/master/related_scripts .
Jarvis, E. D. et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346 , 1320–1331 (2014).
Article ADS CAS PubMed PubMed Central Google Scholar
Prum, R. O. et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526 , 569–573 (2015).
Article ADS CAS PubMed Google Scholar
Kuhl, H. et al. An unbiased molecular approach using 3’-UTRs resolves the avian family-level Tree of Life. Mol. Biol. Evol. 38 , 108–127 (2021).
Article CAS PubMed Google Scholar
Feng, S. et al. Dense sampling of bird diversity increases power of comparative genomics. Nature 587 , 252–257 (2020).
Hinchliff, C. E. et al. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl Acad. Sci. USA 112 , 12764–12769 (2015).
One Thousand Plant Transcriptomes Initiative. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574 , 679–685 (2019).
Jeffroy, O., Brinkmann, H., Delsuc, F. & Philippe, H. Phylogenomics: the beginning of incongruence? Trends Genet. 22 , 225–231 (2006).
Philippe, H. et al. Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol. 9 , e1000602 (2011).
Article CAS PubMed PubMed Central Google Scholar
Schrempf, D. & Szöllősi, G. in Phylogenetics in the Genomic Era (eds Scornavacca, C. et al.) 3.1:1–3.1:23 (2020).
Bravo, G. A. et al. Embracing heterogeneity: coalescing the Tree of Life and the future of phylogenomics. PeerJ 7 , e6399 (2019).
Article PubMed PubMed Central Google Scholar
Wu, S. et al. Genomes, fossils, and the concurrent rise of modern birds and flowering plants in the Late Cretaceous. Proc. Natl Acad. Sci. USA 121 , e2319696121 (2024).
Reddy, S. et al. Why do phylogenomic data sets yield conflicting trees? Data type influences the avian Tree of Life more than taxon sampling. Syst. Biol. 66 , 857–879 (2017).
Braun, E. L. & Kimball, R. T. Data types and the phylogeny of Neoaves. Birds 2 , 1–22 (2021).
Article Google Scholar
Suh, A. The phylogenomic forest of bird trees contains a hard polytomy at the root of Neoaves. Zool. Scr. 45 , 50–62 (2016).
Braun, E. L., Cracraft, J. & Houde, P. in Avian Genomics in Ecology and Evolution: From the Lab into the Wild (ed. Kraus, R. H. S.) 151–210 (Springer International Publishing, 2019).
Hackett, S. J. et al. A phylogenomic study of birds reveals their evolutionary history. Science 320 , 1763–1768 (2008).
Mitchell, K. J., Cooper, A. & Phillips, M. J. Comment on ‘Whole-genome analyses resolve early branches in the tree of life of modern birds’. Science 349 , 1460 (2015).
Cracraft, J. et al. Response to Comment on ‘Whole-genome analyses resolve early branches in the tree of life of modern birds’. Science 349 , 1460 (2015).
Renne, P. R. et al. Time scales of critical events around the Cretaceous-Paleogene boundary. Science 339 , 684–687 (2013).
Hedges, S. B., Parker, P. H., Sibley, C. G. & Kumar, S. Continental breakup and the ordinal diversification of birds and mammals. Nature 381 , 226–229 (1996).
Feduccia, A. ‘Big bang’ for tertiary birds? Trends Ecol. Evol. 18 , 172–176 (2003).
Mayr, G. Paleogene Fossil Birds (Springer International Publishing, 2022).
Claramunt, S. & Cracraft, J. A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci. Adv. 1 , e1501005 (2015).
Article ADS PubMed PubMed Central Google Scholar
Oliveros, C. H. et al. Earth history and the passerine superradiation. Proc. Natl Acad. Sci. USA 116 , 7916–7925 (2019).
McCormack, J. E. et al. A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS ONE 8 , e54848 (2013).
Zwickl, D. J. & Hillis, D. M. Increased taxon sampling greatly reduces phylogenetic error. Syst. Biol. 51 , 588–598 (2002).
Article PubMed Google Scholar
Hedtke, S. M., Townsend, T. M. & Hillis, D. M. Resolution of phylogenetic conflict in large data sets by increased taxon sampling. Syst. Biol. 55 , 522–529 (2006).
Zhang, G. et al. Genomics: bird sequencing project takes off. Nature 522 , 34 (2015).
Chen, M.-Y., Liang, D. & Zhang, P. Phylogenomic resolution of the phylogeny of laurasiatherian mammals: exploring phylogenetic signals within coding and noncoding sequences. Genome Biol. Evol. 9 , 1998–2012 (2017).
Edwards, S. V. et al. Implementing and testing the multispecies coalescent model: a valuable paradigm for phylogenomics. Mol. Phylogenet. Evol. 94 , 447–462 (2016).
Mirarab, S., Nakhleh, L. & Warnow, T. Multispecies coalescent: theory and applications in phylogenetics. Annu. Rev. Ecol. Evol. Syst. 52 , 247–268 (2021).
Suh, A., Smeds, L. & Ellegren, H. The dynamics of incomplete lineage sorting across the ancient adaptive radiation of neoavian birds. PLoS Biol. 13 , e1002224 (2015).
Cracraft, J. in The Howard & Moore Complete Checklist of the Birds of the World. 4th Edition. Volume 1: Non-passerines (eds Dickinson, E. C. & Remsen, J. V.) xxi–xliii (Aves Press, 2013).
Mirarab, S. et al. A region of suppressed recombination misleads neoavian phylogenomics. Proc. Natl Acad. Sci. USA 121 , e2319506121 (2024).
Sayyari, E. & Mirarab, S. Testing for polytomies in phylogenetic species trees using quartet frequencies. Genes 9 , 132 (2018).
Solís-Lemus, C., Yang, M. & Ané, C. Inconsistency of species tree methods under gene flow. Syst. Biol. 65 , 843–851 (2016).
Harvey, M. G. et al. The evolution of a tropical biodiversity hotspot. Science 370 , 1343–1348 (2020).
Moyle, R. G. et al. Tectonic collision and uplift of Wallacea triggered the global songbird radiation. Nat. Commun. 7 , 12709 (2016).
Cloutier, A. et al. Whole-genome analyses resolve the phylogeny of flightless birds (Palaeognathae) in the presence of an empirical anomaly zone. Syst. Biol. 68 , 937–955 (2019).
Nabhan, A. R. & Sarkar, I. N. The impact of taxon sampling on phylogenetic inference: a review of two decades of controversy. Brief. Bioinform. 13 , 122–134 (2012).
Heath, T. A., Hedtke, S. M. & Hillis, D. M. Taxon sampling and the accuracy of phylogenetic analyses. J. Syst. Evol. 46 , 239–257 (2008).
Google Scholar
Lozano-Fernandez, J. A practical guide to design and assess a phylogenomic study. Genome Biol. Evol. 14 , evac129 (2022).
Pick, K. S. et al. Improved phylogenomic taxon sampling noticeably affects nonbilaterian relationships. Mol. Biol. Evol. 27 , 1983–1987 (2010).
Houde, P., Braun, E. L. & Zhou, L. Deep-time demographic inference suggests ecological release as driver of neoavian adaptive radiation. Diversity 12 , 164 (2020).
Yoder, J. B. et al. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23 , 1581–1596 (2010).
Western, D. & Ssemakula, J. Life history patterns in birds and mammals and their evolutionary interpretation. Oecologia 54 , 281–290 (1982).
Article ADS PubMed Google Scholar
Berv, J. S. & Field, D. J. Genomic signature of an avian Lilliput effect across the K-Pg extinction. Syst. Biol. 67 , 1–13 (2018).
Berv, J. S. et al. Molecular early burst associated with the diversification of birds at the K–Pg boundary. Preprint at bioRxiv https://doi.org/10.1101/2022.10.21.513146 (2022).
Ksepka, D. T. et al. Tempo and pattern of avian brain size evolution. Curr. Biol. 30 , 2026–2036 (2020).
Sangster, G. et al. Phylogenetic definitions for 25 higher-level clade names of birds. Avian Res. 13 , 100027 (2022).
Armstrong, J. et al. Progressive Cactus is a multiple-genome aligner for the thousand-genome era. Nature 587 , 246–251 (2020).
Hickey, G., Paten, B., Earl, D., Zerbino, D. & Haussler, D. HAL: a hierarchical format for storing and analyzing multiple genome alignments. Bioinformatics 29 , 1341–1342 (2013).
Springer, M. S. & Gatesy, J. Delimiting coalescence genes (C-genes) in phylogenomic data sets. Genes 9 , 123 (2018).
Mirarab, S. et al. PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J. Comput. Biol. 22 , 377–386 (2015).
Mai, U. & Mirarab, S. TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19 , 272 (2018).
Katoh, K., Misawa, K., Kuma, K.-I. & Miyata, T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30 , 3059–3066 (2002).
Katoh, K. & Standley, D. M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 32 , 1933–1942 (2016).
Faircloth, B. C. PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics 32 , 786–788 (2016).
Morel, B., Kozlov, A. M. & Stamatakis, A. ParGenes: a tool for massively parallel model selection and phylogenetic tree inference on thousands of genes. Bioinformatics 35 , 1771–1773 (2019).
Darriba, D. et al. ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 37 , 291–294 (2020).
Article MathSciNet CAS PubMed Google Scholar
Kozlov, A. M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35 , 4453–4455 (2019).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 , 268–274 (2015).
Anisimova, M. & Gascuel, O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst. Biol. 55 , 539–552 (2006).
Junier, T. & Zdobnov, E. M. The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics 26 , 1669–1670 (2010).
Yin, J., Zhang, C. & Mirarab, S. ASTRAL-MP: scaling ASTRAL to very large datasets using randomization and parallelization. Bioinformatics 35 , 3961–3969 (2019).
Sayyari, E. & Mirarab, S. Fast coalescent-based computation of local branch support from quartet frequencies. Mol. Biol. Evol. 33 , 1654–1668 (2016).
Sayyari, E., Whitfield, J. B. & Mirarab, S. DiscoVista: interpretable visualizations of gene tree discordance. Mol. Phylogenet. Evol. 122 , 110–115 (2018).
Pattengale, N. D., Alipour, M., Bininda-Emonds, O. R. P., Moret, B. M. E. & Stamatakis, A. How many bootstrap replicates are necessary? J. Comput. Biol. 17 , 337–354 (2010).
Álvarez-Carretero, S. et al. A species-level timeline of mammal evolution integrating phylogenomic data. Nature 602 , 263–267 (2022).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37 , 1530–1534 (2020).
Mai, U., Sayyari, E. & Mirarab, S. Minimum variance rooting of phylogenetic trees and implications for species tree reconstruction. PLoS ONE 12 , e0182238 (2017).
Parham, J. F. et al. Best practices for justifying fossil calibrations. Syst. Biol. 61 , 346–359 (2012).
Claramunt, S. CladeDate: calibration information generator for divergence time estimation. Methods Ecol. Evol. 13 , 2331–2338 (2022).
Marshall, C. R. Using the fossil record to evaluate timetree timescales. Front. Genet. 10 , 1049 (2019).
Strauss, D. & Sadler, P. M. Classical confidence intervals and Bayesian probability estimates for ends of local taxon ranges. Math. Geol. 21 , 411–427 (1989).
Azzalini, A. A. The R Package Sn: the Skew-Normal and Related Distributions such as the Skew-T and the SUN (version 2.1.1) (Univ. degli Studi di Padova, 2019).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24 , 1586–1591 (2007).
Thorne, J. L., Kishino, H. & Painter, I. S. Estimating the rate of evolution of the rate of molecular evolution. Mol. Biol. Evol. 15 , 1647–1657 (1998).
Slack, K. E. et al. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol. Biol. Evol. 23 , 1144–1155 (2006).
Yang, Z. & Rannala, B. Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol. Biol. Evol. 23 , 212–226 (2006).
Dos Reis, M., Zhu, T. & Yang, Z. The impact of the rate prior on Bayesian estimation of divergence times with multiple loci. Syst. Biol. 63 , 555–565 (2014).
Bogdanowicz, D., Giaro, K. & Wróbel, B. TreeCmp: comparison of trees in polynomial time. Evol. Bioinform. Online 8 , EBO.S9657 (2012).
Shen, W., Le, S., Li, Y. & Hu, F. SeqKit: a cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 11 , e0163962 (2016).
Haag, J., Höhler, D., Bettisworth, B. & Stamatakis, A. From easy to hopeless-predicting the difficulty of phylogenetic analyses. Mol. Biol. Evol. 39 , msac254 (2022).
International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432 , 695–716 (2004).
Elferink, M. G., van As, P., Veenendaal, T., Crooijmans, R. P. M. A. & Groenen, M. A. M. Regional differences in recombination hotspots between two chicken populations. BMC Genet. 11 , 11 (2010).
Foster, P. G. Modeling compositional heterogeneity. Syst. Biol. 53 , 485–495 (2004).
Duchêne, D. A., Duchêne, S. & Ho, S. Y. W. New statistical criteria detect phylogenetic bias caused by compositional heterogeneity. Mol. Biol. Evol. 34 , 1529–1534 (2017).
Duchêne, D. A., Mather, N., Van Der Wal, C. & Ho, S. Y. W. Excluding loci with substitution saturation improves inferences from phylogenomic data. Syst. Biol. 71 , 676–689 (2022).
Rivas-González, I. et al. Pervasive incomplete lineage sorting illuminates speciation and selection in primates. Science 380 , eabn4409 (2023).
Mendes, F. K. & Hahn, M. W. Gene tree discordance causes apparent substitution rate variation. Syst. Biol. 65 , 711–721 (2016).
Walker, J. F., Smith, S. A., Hodel, R. G. J. & Moyroud, E. Concordance-based approaches for the inference of relationships and molecular rates with phylogenomic data sets. Syst. Biol. 71 , 943–958 (2022).
Pagel, M. Inferring the historical patterns of biological evolution. Nature 401 , 877–884 (1999).
Tobias, J. A. et al. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 25 , 581–597 (2022).
Ho, L. S. T. & Ané, C. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63 , 397–408 (2014).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3 , 217–223 (2012).
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- Image & Use Policy
- Translations
UC MUSEUM OF PALEONTOLOGY
Understanding Evolution
Your one-stop source for information on evolution
Macroevolution through evograms
The origin of birds.
The discovery that birds evolved from small carnivorous dinosaurs of the Late Jurassic was made possible by recently discovered fossils from China, South America, and other countries, as well as by looking at old museum specimens from new perspectives and with new methods. The hunt for the ancestors of living birds began with a specimen of Archaeopteryx , the first known bird, discovered in the early 1860s. Like birds, it had feathers along its arms and tail, but unlike living birds, it also had teeth and a long bony tail. Furthermore, many of the bones in Archaeopteryx ‘s hands, shoulder girdles, pelvis, and feet were distinct, not fused and reduced as they are in living birds. Based on these characteristics, Archaeopteryx was recognized as an intermediate between birds and reptiles; but which reptiles?
In the 1970s, paleontologists noticed that Archaeopteryx shared unique features with small carnivorous dinosaurs called theropods. All the dinosaur groups on this evogram, except the ornithischian dinosaurs, are theropods. Based on their shared features, scientists reasoned that perhaps the theropods were the ancestors of birds. When paleontologists built evolutionary trees to study the question, they were even more convinced. The birds are simply a twig on the dinosaurs’ branch of the tree of life.
As birds evolved from these theropod dinosaurs, many of their features were modified. However, it’s important to remember that the animals were not “trying” to be birds in any sense. In fact, the more closely we look, the more obvious it is that the suite of features that characterize birds evolved through a complex series of steps and served different functions along the way.
Take feathers, for example. Small theropods related to Compsognathus (e.g., Sinosauropteryx ) probably evolved the first feathers. These short, hair-like feathers grew on their heads, necks, and bodies and provided insulation. The feathers seem to have had different color patterns as well, although whether these were for display, camouflage, species recognition, or another function is difficult to tell.

In theropods even more closely related to birds, like the oviraptorosaurs, we find several new types of feathers. One is branched and downy, as pictured below. Others have evolved a central stalk, with unstructured branches coming off it and its base. Still others (like the dromaeosaurids and Archaeopteryx ) have a vane-like structure in which the barbs are well-organized and locked together by barbules. This is identical to the feather structure of living birds.

Another line of evidence comes from changes in the digits of the dinosaurs leading to birds. The first theropod dinosaurs had hands with small fifth and fourth digits and a long second digit. As the evogram shows, in the theropod lineage that would eventually lead to birds, the fifth digit (e.g., as seen in Coelophysoids) and then the fourth (e.g., as seen in Allosaurids) were completely lost. The wrist bones underlying the first and second digits consolidated and took on a semicircular form that allowed the hand to rotate sideways against the forearm. This eventually allowed birds’ wing joints to move in a way that creates thrust for flight.
The functions of feathers as they evolved have long been debated. As we have seen, the first, simplest, hair-like feathers obviously served an insulatory function. But in later theropods, such as some oviraptorosaurs, the feathers on the arms and hands are long, even though the forelimbs themselves are short. What did these animals do with long feathers on short arms? One suggestion comes from some remarkable fossils of oviraptorosaurs preserved in the Cretaceous sediments of the Gobi Desert. The skeleton of the animal is hunched up on a nest of eggs, like a brooding chicken. The hands are spread out over the eggs as if to shelter them. So perhaps these feathers served the function of warming the eggs and shielding them from harm.
Birds after Archaeopteryx continued evolving in some of the same directions as their theropod ancestors. Many of their bones were reduced and fused, which may have helped increase the efficiency of flight. Similarly, the bone walls became even thinner, and the feathers became longer and their vanes asymmetrical, probably also improving flight. The bony tail was reduced to a stump, and a spray of feathers at the tail eventually took on the function of improving stability and maneuverability. The wishbone, which was present in non-bird dinosaurs, became stronger and more elaborate, and the bones of the shoulder girdle evolved to connect to the breastbone, anchoring the flight apparatus of the forelimb. The breastbone itself became larger, and evolved a central keel along the midline of the breast which served to anchor the flight muscles. The arms evolved to be longer than the legs, as the main form of locomotion switched from running to flight, and teeth were lost repeatedly in various lineages of early birds. The ancestor of all living birds lived sometime in the Late Cretaceous, and in the 65 million years since the extinction of the rest of the dinosaurs, this ancestral lineage diversified into the major groups of birds alive today.
- Teaching Resources
Teach your students about the evolution of birds:
- The evolution of flight in birds , an online investigation for grades 9-12.
Jaws to ears in the ancestors of mammals
The emergence of humans
Subscribe to our newsletter
- Teaching resource database
- Correcting misconceptions
- Conceptual framework and NGSS alignment
- Image and use policy
- Evo in the News
- The Tree Room
- Browse learning resources
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The Origin and Diversification of Birds
Affiliations.
- 1 School of GeoSciences, University of Edinburgh, Grant Institute, King's Buildings, James Hutton Road, Edinburgh EH9 3FE, UK. Electronic address: [email protected].
- 2 Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China. Electronic address: [email protected].
- 3 Department of Neurobiology, Duke University Medical Center, Durham, NC 27710, USA; Howard Hughes Medical Institute, Chevy Chase, MD 20815, USA. Electronic address: [email protected].
- PMID: 26439352
- DOI: 10.1016/j.cub.2015.08.003
Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two decades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dinosaurs during the Jurassic (around 165-150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous, becoming capable fliers with supercharged growth rates, but were decimated at the end-Cretaceous extinction alongside their close dinosaurian relatives. After the mass extinction, modern birds (members of the avian crown group) explosively diversified, culminating in more than 10,000 species distributed worldwide today.
Copyright © 2015 Elsevier Ltd. All rights reserved.
PubMed Disclaimer
Similar articles
- Dental Disparity and Ecological Stability in Bird-like Dinosaurs prior to the End-Cretaceous Mass Extinction. Larson DW, Brown CM, Evans DC. Larson DW, et al. Curr Biol. 2016 May 23;26(10):1325-33. doi: 10.1016/j.cub.2016.03.039. Epub 2016 Apr 21. Curr Biol. 2016. PMID: 27112293
- Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. Benson RB, Campione NE, Carrano MT, Mannion PD, Sullivan C, Upchurch P, Evans DC. Benson RB, et al. PLoS Biol. 2014 May 6;12(5):e1001853. doi: 10.1371/journal.pbio.1001853. eCollection 2014 May. PLoS Biol. 2014. PMID: 24802911 Free PMC article.
- Theropod courtship: large scale physical evidence of display arenas and avian-like scrape ceremony behaviour by Cretaceous dinosaurs. Lockley MG, McCrea RT, Buckley LG, Lim JD, Matthews NA, Breithaupt BH, Houck KJ, Gierliński GD, Surmik D, Kim KS, Xing L, Kong DY, Cart K, Martin J, Hadden G. Lockley MG, et al. Sci Rep. 2016 Jan 7;6:18952. doi: 10.1038/srep18952. Sci Rep. 2016. PMID: 26741567 Free PMC article.
- An exceptionally preserved Lower Cretaceous ecosystem. Zhou Z, Barrett PM, Hilton J. Zhou Z, et al. Nature. 2003 Feb 20;421(6925):807-14. doi: 10.1038/nature01420. Nature. 2003. PMID: 12594504 Review.
- The Paleogene fossil record of birds in Europe. Mayr G. Mayr G. Biol Rev Camb Philos Soc. 2005 Nov;80(4):515-42. doi: 10.1017/S1464793105006779. Biol Rev Camb Philos Soc. 2005. PMID: 16221327 Review.
- The Evolution of Ultraconserved Elements in Vertebrates. Cummins M, Watson C, Edwards RJ, Mattick JS. Cummins M, et al. Mol Biol Evol. 2024 Jul 3;41(7):msae146. doi: 10.1093/molbev/msae146. Mol Biol Evol. 2024. PMID: 39058500 Free PMC article.
- Earliest evidence of avian primary feather moult. Wang X, O'Connor J, Zheng X, Wang Y, Kiat Y. Wang X, et al. Biol Lett. 2024 Jun;20(7):20240106. doi: 10.1098/rsbl.2024.0106. Epub 2024 Jul 3. Biol Lett. 2024. PMID: 38955226
- Evolution of bird sex chromosomes: a cytogenomic approach in Palaeognathae species. Setti PG, Deon GA, Zeni Dos Santos R, Goes CAG, Garnero ADV, Gunski RJ, de Oliveira EHC, Porto-Foresti F, de Freitas TRO, Silva FAO, Liehr T, Utsunomia R, Kretschmer R, de Bello Cioffi M. Setti PG, et al. BMC Ecol Evol. 2024 Apr 23;24(1):51. doi: 10.1186/s12862-024-02230-5. BMC Ecol Evol. 2024. PMID: 38654159 Free PMC article.
- Inferring aerial behavior in Mesozoic dinosaurs: Implications and uncertainties. Xu X. Xu X. Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2401482121. doi: 10.1073/pnas.2401482121. Epub 2024 Mar 11. Proc Natl Acad Sci U S A. 2024. PMID: 38466860 Free PMC article. No abstract available.
- Functional constraints on the number and shape of flight feathers. Kiat Y, O'Connor JK. Kiat Y, et al. Proc Natl Acad Sci U S A. 2024 Feb 20;121(8):e2306639121. doi: 10.1073/pnas.2306639121. Epub 2024 Feb 12. Proc Natl Acad Sci U S A. 2024. PMID: 38346196 Free PMC article.
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Elsevier Science
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Tree of Life for modern birds revealed

Professor Simon Ho (seated) with Dr Jacqueline Nguyen at the Australian Museum. Photo: James Alcock
In a world first, a team of international scientists including three Australians, Al-Aabid Chowdhury and Professor Simon Ho from University of Sydney, and Dr Jacqueline Nguyen from Australian Museum and Flinders University, have determined the family tree of modern birds and pinpointed the timing of their evolution.
Their findings have been published today in Nature .
The largest study ever undertaken of modern bird genomes, the scientists combined genomic data of more than 360 bird species with data from nearly 200 bird fossils to reconstruct the most well-supported Tree of Life for modern birds.
The research revealed that most modern bird groups appeared within a very small evolutionary window of only five million years. These findings support the hypothesis that birds made the most of opportunities after an asteroid struck earth 66 million years ago wiping out the dinosaurs.
The comprehensive study was led by Assistant Professor Josefin Stiller from the University of Copenhagen, along with Associate Professor Siavash Mirarab from the University of California, San Diego and Professor Guojie Zhang from Zhejiang University.
“Our study has resolved some previous disputes about the bird family tree and added new nuance to the textbook knowledge of bird evolution,” Assistant Professor Stiller said.
Earlier studies had already established that the 10,000 species of living birds form three major groups. About 500 species belong to the flightless ratites group or the landfowl-waterfowl group, however all other birds form a third large and diverse group called Neoaves .
The latest study has been able to establish deeper understanding of relationships in the Neoaves group, which itself contains 10 major sub-groups of birds. These include the colourfully named ‘Magnificent Seven’, including cuckoos, doves, and flamingos, along with three ‘orphan’ groups of birds whose ancestry has long been uncertain.
Professor Ho, who specialises in evolutionary biology at the University of Sydney, said the research has worked out the evolutionary relationships of the major bird groups.
“With such a huge amount of genome data, our study has been able to provide the clearest picture of the bird family tree so far, particularly among the ‘Magnificent Seven’ and three ‘orphan’ bird groups, which make up 95 percent of bird species,” Professor Ho said.

The bird tree of life, based on the genomes of 363 bird species. The major bird groups are colour-coded in the tree. Paintings: Jon Fjeldså, Natural History Museum Denmark, University of Copenhagen
Australian Museum and Flinders University avian palaeontologist, Dr Jacqueline Nguyen, said the fossil information was used to work out the timescale of the bird family tree.
“By combining evidence from nearly 200 bird fossils, we were able to pinpoint an extremely important period of bird diversification that happened immediately after the extinction of the dinosaurs,” Dr Nguyen said.
The genomes also reveal a new grouping of birds that the researchers have named ‘Elementaves’, inspired by the four ancient elements of earth, air, water and fire. The group includes birds that are successful on land, in the sky, and in water. Some birds have names relating to the sun, representing fire. Penguins, pelicans, swifts, hummingbirds and shorebirds are among the birds that have been placed in Elementaves.
Two of the most well-known groups of birds in Australia, the passerines (songbirds and relatives) and parrots, share a very close relationship. Songbirds include familiar birds such as magpies, ravens, finches, honeyeaters and fairy-wrens. They originated in Australia about 50 million years ago and have become the most successful group of birds, making up nearly half of all bird species worldwide.
Despite the enormous scale of the latest genome study, there is one mystery that continues. The researchers were unable to work out the relationships of the hoatzin, a distinctive bird that is only found in South America and is the sole survivor of its entire lineage.
The findings are the outcome of nearly a decade of research involving scientists from across the globe working together on the Bird 10,000 Genomes Project (B10K), which aims to sequence the complete genomes of every living bird species.
Chief scientist and Director of the Australian Museum Research Institute, Professor Kris Helgen, said that genomic tools have precipitated one of the great revolutions in biological sciences.
“The global scientific community has come together to champion impressive genome projects like Bird 10K. Efforts like these can address long-standing questions about evolution, in this case for all living species of birds. They do this by drawing on new genetics techniques, expertise on anatomy and the fossil record, and carefully curated DNA samples, which are stored behind-the-scenes in the collections of natural history museums in Australia and around the world,” Professor Helgen said.
Declaration
Research funds were received from multiple organisations, including the Australian Research Council, European Union, Chinese Academy of Sciences, National Natural Science Foundation of China, Klaus Tschira Foundation, Natural Scienes and Engineering Research Council of Canada, US National Science Foundation. For a full list of funders, see the paper in Nature .
Simon Ho research lab
Read the study, media contact.
- +61 2 8627 6433
- [email protected]
Related articles
Wrasses dazzle: how fairy wrasses got their flamboyant colours, how an invasive bee colony defied a genetic bottleneck, molecular ecology, evolution, and phylogenetics lab.
We are interested in various aspects of molecular ecology and evolutionary biology, including phylogenetic methods, molecular clocks, molecular systematics, phylogeography, and environmental DNA.
Accept cookies?
We use cook ies to give you the best online experience and to show personalised content and marketing. We use them to improve our website and content as well as to tailor our digital advertising on third-party platforms. You can change your preferences at any time.
Popular search terms:
- British wildlife
- Wildlife Photographer of the Year
- Explore the Museum
Anthropocene
British Wildlife
Collections
Human evolution
What on Earth?

The most detailed bird evolutionary tree to date has found that the tiny hummingbirds are more closely related to massive albatrosses than previously thought ©Rosalie Kreulen/Shutterstock
During Beta testing articles may only be saved for seven days.
Create a list of articles to read later. You will be able to access your list from any article in Discover.
You don't have any saved articles.
Most detailed bird evolutionary tree reveals new and surprising relationships
Researchers have created the most detailed evolutionary tree of birds to date.
It proposes an entirely new group of birds that connects the smallest flying birds to the largest, as well as showing in finer detail how birds diversified rapidly after the extinction of the dinosaurs.
The smallest flying birds might be more closely related to the largest than previously thought.
A new genetic analysis of all the families of birds has found that hummingbirds and albatrosses form part of an entirely new group of birds, whilst it has also revealed that as opposed to other studies the eagles and owls are actually each other’s closest relatives.
The researchers have used the genetic information from 363 species of birds covering 92% of all bird families to piece together an immense family tree. This is the most complete, highest-resolution evolutionary tree for birds created to date.
Professor Guojie Zhang is a professor on evolutionary biology at Zhejiang University and senior author of this research. He says, “the amount of data is vastly increased from before, with exceptionally wide taxonomic coverage and detailed genomic sequence information.”
“Eventually we want to obtain sequence data on all living species of birds. The combination of genomic, ecological and behavioural data from thousands of bird species will be essential for combating diseases like avian influenza, and they will be a treasure trove for conserving birds worldwide.”
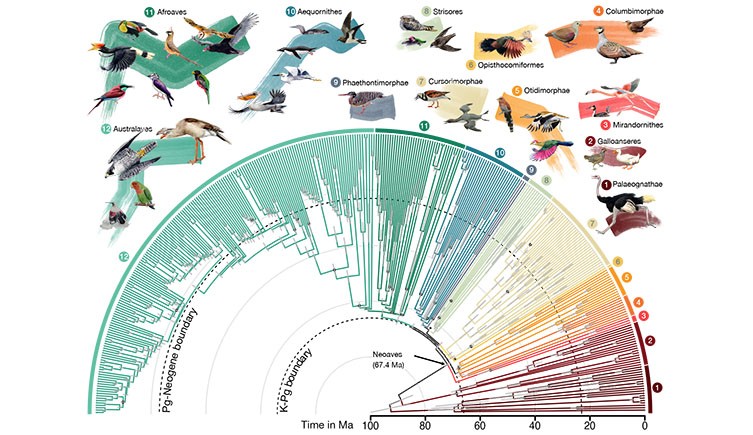
This latest study has used the genetic data from 363 species of birds covering 92% of all bird families to piece together an immense family tree ©Stiller et al. 2023
The research is part of the Bird 10,000 Genomes (B10K) Consortium , which aims to sequence the genomes of every single species of bird. The study was led by Zhang together with Assistant Professor Josefin Stiller from the University of Copenhagen and Associate Professor Siavash Mirarab from the University of California, San Diego, and has been published in the journal Nature .
In the wake of the dinosaurs
Birds are an extraordinarily diverse group of animals. They can be found on every single continent, living in environments ranging from the driest desert to the wettest rainforest.
Within these habitats, they have further diversified to exploit a massive number of different niches. There are birds that dive hundreds of metres beneath the waves for fish, some which crack the hardest of nuts, and those that have evolved in tight association with plants that provide their high-sugar nectar. Others still have specialised in eating other birds, or cleaning up the carcasses left behind by larger mammals.
While the general relationships between birds, and those between species within specific groups, have been fairly well studied and understood, where exactly many groups of birds sit with relation to each other has been surprisingly difficult to figure out.
For example, the curious hoatzin is a species of bird found living along the banks of the Amazon River. It is so evolutionarily distinct, living entirely on a diet of leaves and producing chicks that still retain a claw on their wings, that it forms its own distinct group which scientists have long struggled to place. It is so unusual, the hoatzin doesn’t seem to be obviously related to any other group of birds.

While previously placed on separate evolutionary branches, the research has placed the owls as a sister group to the eagles and buzzards ©Alan Tunnicliffe/Shutterstock
This is because there is a point in time when there was a flash of evolution within birds, a flurry of diversification at about the same time as when the asteroid that wiped out their dinosaur cousins hit. But even then, there has still been a debate as to whether this burst of evolution occurred before or after the dinosaur extinction event.
The much improved precision in dating from this new paper seems to have finally settled this.
Dr Martin Stervander is the Senior Curator of Birds at National Museums Scotland, and a Scientific Associate at the Natural History Museum.
“The cool thing with the dating is that we can actually show that almost all of that diversification happened after the meteorite,” explains Martin, who was involved in this latest paper. “It looks like almost all of the diversification happened when a lot of the competitors for niches and space were wiped out, but our lovely winged friends survived.”
This period of extremely rapid evolution, as the birds moved into new environments and niches left vacant by the dinosaurs, is the reason why it has been hard for biologists to tease out which groups – such as the hoatzin – are most closely related to each other.
But the input of vast amounts of genetic data from right across the range of bird families is finally starting to reveal some of these relationships. One of the biggest changes is the creation of an entirely new group of birds.
Meet the elemental birds
The research has found that a seemingly diverse group of birds actually cluster together in one new group.
This includes many species you might expect to be related, such as pelicans, penguins and albatrosses , but also a whole bunch of other perhaps more unusual species, including the swifts, hummingbirds and those pesky hoatzins. It is the surprisingly diverse lifestyles of these birds – including the smallest and largest flying birds – that gave inspiration to their new name.

Hoatzins have been something of an evolutionary mystery, but this latest research has now placed them in an entirely new group called the Elementaves ©Morten Ross/Shutterstock
“We named this new group Elementaves ,” explains Martin. “This is because the birds within this group have diversified into aquatic, terrestrial, and aerial niches – including some of our most impressive specialists like penguins and swifts – corresponding to the classical elements of water, earth, and wind.”
“But what about fire? Well, sunbittern and tropicbirds of Phaetontimorphae have names derived from the sun, which is a big ball of fire.”
The other perhaps logical – but no less surprising – reshuffling from the paper involved the eagles and owls. Despite having similar lifestyles and adaptations, albeit working different shifts, the birds have traditionally these have been placed in separate branches of the family tree. They have now been brought together.
“Another new finding is that diurnal raptors like eagles and buzzards indeed make up the sister lineage to owls, the raptors of the night,” says Martin.
Whilst this latest work has produced the most detailed bird evolutionary tree to date, that’s not to say it won’t change again. The B10K project aims to sequence the genomes of every single species of bird, and there is no saying how this might reveal yet more surprises in the evolution of birds.

Birds: Brilliant and Bizarre
Unravel the epic story of birds, from surviving a mass extinction event to inhabiting every continent on Earth. Discover the secrets to their success and their surprising and often shocking tactics for survival.

Just how weird can the natural world be?
Discover more
Bird evolution slowed down after the dinosaurs died
The skull shape of birds is just a tiny fraction of the diversity that would have been seen in their dinosaur ancestors.


Bird beaks reveal the changeable nature of evolution
The Natural History Museum’s collections are helping scientists to get a handle on how evolution works.

If you're a bird, you aren't what you eat
A new paper is shedding light on the relationship between a bird's diet and how it looks.

Penguins are some of the slowest-evolving birds in the world
The evolution of penguins has been laid out in unparalleled detail, revealing how they came into being.
Don't miss a thing
Receive email updates about our news, science, exhibitions, events, products, services and fundraising activities. We may occasionally include third-party content from our corporate partners and other museums. We will not share your personal details with these third parties. You must be over the age of 13. Privacy notice .
Follow us on social media
Our systems are now restored following recent technical disruption, and we’re working hard to catch up on publishing. We apologise for the inconvenience caused. Find out more: https://www.cambridge.org/universitypress/about-us/news-and-blogs/cambridge-university-press-publishing-update-following-technical-disruption
We use cookies to distinguish you from other users and to provide you with a better experience on our websites. Close this message to accept cookies or find out how to manage your cookie settings .
Login Alert
- > Journals
- > Biological Reviews
- > Volume 73 Issue 1
- > The origin and early evolution of birds

Article contents
The origin and early evolution of birds.
Published online by Cambridge University Press: 01 February 1998
Birds evolved from and are phylogenetically recognized as members of the theropod dinosaurs; their first known member is the Late Jurassic Archaeopteryx , now represented by seven skeletons and a feather, and their closest known non-avian relatives are the dromaeosaurid theropods such as Deinonychus . Bird flight is widely thought to have evolved from the trees down, but Archaeopteryx and its outgroups show no obvious arboreal or tree-climbing characters, and its wing planform and wing loading do not resemble those of gliders. The ancestors of birds were bipedal, terrestrial, agile, cursorial and carnivorous or omnivorous. Apart from a perching foot and some skeletal fusions, a great many characters that are usually considered ‘avian’ (e.g. the furcula, the elongated forearm, the laterally flexing wrist and apparently feathers) evolved in non-avian theropods for reasons unrelated to birds or to flight. Soon after Archaeopteryx , avian features such as the pygostyle, fusion of the carpometacarpus, and elongated curved pedal claws with a reversed, fully descended and opposable hallux, indicate improved flying ability and arboreal habits. In the further evolution of birds, characters related to the flight apparatus phylogenetically preceded those related to the rest of the skeleton and skull. Mesozoic birds are more diverse and numerous than thought previously and the most diverse known group of Cretaceous birds, the Enantiornithes, was not even recognized until 1981. The vast majority of Mesozoic bird groups have no Tertiary records: Enantiornithes, Hesperornithiformes, Ichthyornithiformes and several other lineages disappeared by the end of the Cretaceous. By that time, a few Linnean ‘Orders’ of extant birds had appeared, but none of these taxa belongs to extant ‘families’, and it is not until the Paleocene or (in most cases) the Eocene that the majority of extant bird ‘Orders’ are known in the fossil record. There is no evidence for a major or mass extinction of birds at the end of the Cretaceous, nor for a sudden ‘bottleneck’ in diversity that fostered the early Tertiary origination of living bird ‘Orders’.
Access options

This article has been cited by the following publications. This list is generated based on data provided by Crossref .
- Google Scholar
View all Google Scholar citations for this article.
Save article to Kindle
To save this article to your Kindle, first ensure [email protected] is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about saving to your Kindle .
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
- Volume 73, Issue 1
- KEVIN PADIAN (a1) and LUIS M. CHIAPPE (a2)
- DOI: https://doi.org/10.1017/S0006323197005100
Save article to Dropbox
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox .
Save article to Google Drive
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive .
Reply to: Submit a response
- No HTML tags allowed - Web page URLs will display as text only - Lines and paragraphs break automatically - Attachments, images or tables are not permitted
Your details
Your email address will be used in order to notify you when your comment has been reviewed by the moderator and in case the author(s) of the article or the moderator need to contact you directly.
You have entered the maximum number of contributors
Conflicting interests.
Please list any fees and grants from, employment by, consultancy for, shared ownership in or any close relationship with, at any time over the preceding 36 months, any organisation whose interests may be affected by the publication of the response. Please also list any non-financial associations or interests (personal, professional, political, institutional, religious or other) that a reasonable reader would want to know about in relation to the submitted work. This pertains to all the authors of the piece, their spouses or partners.
- Paleontology
- Paleobiology
Evolution. How birds became birds
- August 2014
- Science 345(6196):508-9
- 345(6196):508-9

- University of Bristol
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- John Sibbick

- Toru Shimizu

- Barry Gordon Lovegrove
- Lee Michael S.Y.

- Sofia M. Sinitsa

- Jakob Vinther
- Richard O. Prum

- Stuart L. Kearns

- Zhonghe Zhou

- Joseph Felsenstein
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
Bird Evolution Was Rapid, and Happened Early On, Studies Say
How birds evolved is a complex question that has puzzled biologists for decades. A spate of papers published today in the journal Science provides the clearest picture to date of the avian family tree. By mapping the genomes of 45 bird species for the first time (and using three previously mapped genomes), the researchers were able to more closely trace relationships between species—and to confirm that birds underwent an early, rapid, ‘big bang’ that led to the evolution of the more than 10,000 species we have today.
Over 200 scientists from 20 countries joined together to form the Avian Phylogenomics Consortium, the umbrella organization for the research. The project spanned four years, and yielded 28 separate research papers, all published this week.
“How do birds relate to each other? This was our fundamental first question,” says Tom Gilbert , lead author on one of the studies, and head of the section for evolutionary genomics at the Natural History Museum of Denmark, during Science’s press conference.
Most modern-day bird species—95 percent of them—reside on a single branch (or “clade”) of the avian family tree called Neoaves that exists close to the base. The genetic differentiation within this group, however, isn’t very distinct, so ornithologists have been stumped about exactly how the various lineages within it split off as today’s species evolved.
“There were parts of the tree that were pretty well resolved before this, but when you looked at the base it was very difficult to test relationships,” says Edward Braun , associate professor of biology at the University of Florida. There were so many different theories for how the original splits happened that it was very difficult to rigorously analyze any one idea. Additionally, avian evolution research has previously relied on smaller gene sets, which caused data inconsistencies, further inhibiting any consensus on what the tree looked like.
For this body of work, the researchers changed the game by using full genome mapping of 48 species, which together encompass all the bird lineages alive today. “What’s impressive here is the number of different species that they’ve done,” says Jon Slate , an evolutionary geneticist at the University of Sheffield who has worked on bird genome projects in the past, but was not involved in the research. “There are almost five-fold as many birds sequenced now.” With this data, the APC were able to reconstruct the avian family tree in unprecedented detail. “This is the biggest DNA sequenced tree ever generated. In this case it’s 30 billion base pairs long,” says Erich Jarvis , an associate professor of avian neurobiology at Duke University School of Medicine.
What Hurt Dinos Helped Birds
In addition to reconstructing a fuller avian tree, the mapping also helped settle a longstanding controversy over when the mass expansion of Neoaves occurred. Past research has suggested this group evolved somewhere between 10 to 80 million years before the mass extinction that killed the dinosaurs. But the genome project allowed the APC to pinpoint the evolutionary development of Neoaves to about 66 million years ago, meaning the origin of all bird species today likely happened right around the time of the mass extinction that wiped out dinosaurs at the end of the Mezozoic Era.
“We’re suggesting [the expansion] occurred right at that time, with only a few lineages surviving the mass extinction, and then giving rise to all these Neoaves groups within the last 66 million years,” Jarvis says. The huge species gap left by the dinosaurs likely created an accommodating environment for the widespread expansion of bird species—one that happened very rapidly in evolutionary terms, Jarvis says.
“All modern orders formed from this radiation within a 10 to 15 million year period, around 50 million years ago,” he adds.
The reworked avian tree also reveals some unexpected relationships between species that were unknown until recently: Flamingoes are related to pigeons, for example, and even though falcons seem similar to eagles, they’re actually more closely linked to parrots and songbirds.
The level of detail available in the new avian tree will allow researchers to delve more deeply into avian biology going forward. “We can dig into very specific bird traits and look at the genetic basis of them; things like flight, feathers, vision, olfaction, sexual selection,” Gilbert says. “We hope we can start addressing even more exciting questions—like whether we can infer things about dinosaurs.”
The project will be ongoing, Jarvis says, and could ultimately help researchers map other datasets, including insects, mammals, and plants. Researchers say they’re already seeing noticeable parallels between the avian and mammal genomes.
“The findings will certainly help people to decide which questions to ask,” says Slate. “This, in a sense, is big toolbox that’s being described.”

Pledge to stand with Audubon to call on elected officials to listen to science and work towards climate solutions.
April 16, 2024
13 min read
Why Feathers Are One of Evolution’s Cleverest Inventions
Fossil and living birds reveal the dazzling biology of feathers
By Michael B. Habib

Bar-tailed Godwits undertake the longest nonstop migration of any land bird in the world. rockptarmigan/Getty Images
I n October 2022 a bird with the code name B6 set a new world record that few people outside the field of ornithology noticed. Over the course of 11 days, B6, a young Bar-tailed Godwit, flew from its hatching ground in Alaska to its wintering ground in Tasmania, covering 8,425 miles without taking a single break. For comparison, there is only one commercial aircraft that can fly that far nonstop, a Boeing 777 with a 213-foot wingspan and one of the most powerful jet engines in the world. During its journey, B6—an animal that could perch comfortably on your shoulder—did not land, did not eat, did not drink and did not stop flapping , sustaining an average ground speed of 30 miles per hour 24 hours a day as it winged its way to the other end of the world.
Many factors contributed to this astonishing feat of athleticism—muscle power, a high metabolic rate and a physiological tolerance for elevated cortisol levels, among other things. B6’s odyssey is also a triumph of the remarkable mechanical properties of some of the most easily recognized yet enigmatic structures in the biological world: feathers. Feathers kept B6 warm overnight while it flew above the Pacific Ocean. Feathers repelled rain along the way. Feathers formed the flight surfaces of the wings that kept B6 aloft and drove the bird forward for nearly 250 hours without failing.
One might expect that, considering all the time humans have spent admiring, using and studying feathers, we would know all their tricks by now. Yet insights into these marvelous structures continue to emerge. Over the past decade other researchers and I have been taking a fresh look at feathers. Collectively we have made surprising new discoveries about almost every aspect of their biology, from their evolutionary origins to their growth, development and aerodynamics.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Among the creatures we share the planet with today, only birds have feathers. It makes sense, then, that for centuries scientists considered feathers a unique feature of birds. But starting in the 1990s, a series of bombshell fossil finds established that feathers were widespread among several lineages of the bipedal, carnivorous dinosaurs known as theropods and that birds had inherited these structures from their theropod ancestors. The discovery of feathered nonbird dinosaurs sent researchers scrambling to understand the origin and evolution of feathers, especially their role in the dawn of flight. We now know many dinosaurs had feathers, and protofeathers probably go all the way back to the common ancestor of dinosaurs and their flying reptile cousins, the pterosaurs . Bristles, fuzzy coverings, and other relatively simple featherlike structures probably decorated a wide array of dinosaurs—many more than we have been lucky enough to find preserved as fossils.

Feathers, such as those of the Lesser Flamingo shown here, are products of hundreds of millions of years of evolution. Robert Clark
The feathers on nonbird dinosaurs were not limited to bristles and fuzz, however. The flat, broad, flight-enabling feathers we see across most of the wings and much of the body surface of living birds are called pennaceous feathers. (Fun fact: these are the feathers people used to make into quills for writing, hence the word “pen.”) It turns out that these feathers, too, appeared before birds. In fact, there is an entire group of dinosaurs comprising birds as well as species such as Velociraptor that takes its name from these very feathers: the pennaraptoran clade. Fossils of early pennaraptorans show that they had feathery coverings that would have looked essentially modern at a quick glance.
The flight capacity of these early pennaraptorans has been hotly contested. Some species were clearly not fliers, given the small size of their “wings” relative to their large bodies. For those animals, pennaceous feathers were probably display pieces. But other pennaraptorans, such as the small, four-winged, forest-dwelling Microraptor , are trickier to interpret. Many of the arguments about whether this creature could fly have centered on something called vane asymmetry. The two flat “blades” of a feather on either side of the main shaft are called vanes. In living birds that fly, the feathers that arise from the hand, known as the primaries, have asymmetrical vanes: the leading vane is narrower than the trailing one. It stood to reason that vane asymmetry was important for flight. And because fossils of Microraptor and its kin show asymmetrical feathers, some researchers argued, these animals must have been able to fly.
Maria Amorette Klos
Recent work by flight biomechanics experts, including me, has overturned this received wisdom about feather vane asymmetry. Our research shows that feather shape is largely optimized to allow the feather to twist and bend in sophisticated ways that greatly enhance flight performance. Merely being anatomically asymmetrical doesn’t mean much. What matters is that the feather is aerodynamically asymmetrical, and for this to be the case, the vane asymmetry must be at least three to one—that is, the trailing blade needs to be three times wider than the leading one. Below this ratio, the feather twists in a destabilizing rather than stabilizing way during flight.
Early pennaraptorans such as Microraptor didn’t have aerodynamically asymmetrical feathers. But that doesn’t mean they couldn’t fly. The tendency to twist (whether in a stabilizing or a destabilizing fashion) is only relevant if the feathers are separated enough to do so. Keeping feathers in a wing tip tight and overlapping makes them stable, even if they’re not asymmetrical. Asymmetry matters only if the flier spreads its primaries apart in flight like many modern raptors do—a feature called slotting. So Microraptor and its kin could probably use flapping flight, but their wing shape was necessarily different from that of today’s forest-dwelling birds of prey. Specifically, Microraptor had relatively long, narrow wings with tight, unslotted wing tips—anatomically distinct from the wings of Cooper’s Hawks and other modern-day forest hawks but aerodynamically similar.

After considering these findings on vane asymmetry, as well as new data on flight muscles in near-bird dinosaurs, a group of researchers (of which I was the senior biophysicist) led by Michael Pittman of the Chinese University of Hong Kong recently concluded that powered flight—that is, flapping flight rather than gliding flight—probably evolved multiple times in dinosaurs, with just one of those lineages surviving to the present in the form of birds. Yet only in birds did flight feathers attain the degree of shape-shifting we see today. That ability of feathers to twist in just the right way is what enabled slotting, which makes the wing much more efficient at low flight speeds. In essence, a slotted wing behaves as if it is longer and narrower than it is anatomically. Slotting also makes the wing tip very resistant to stall, whereby the airflow separates from the wing, causing a precipitous loss of the lift that keeps the bird in the air. It’s a vital adaptation that underpins an array of aerial acrobatics.
Birds typically need long, narrow wings to soar efficiently—seabirds such as albatrosses and petrels are perfect examples. The advent of wing-tip slots made it possible to soar with broader wings, paving the way for evolution of a diversity of broad-winged soarers, including vultures and hawks. The aerodynamic advantages of slotting also permit the explosive flight performance of sprinters such as grouses, which spend most of their time on the ground but burst into flight for a short distance when startled. And wing-tip slots provide much greater maneuverability for a wide array of birds that live in forests and other cluttered environments, from songbirds to toucans. In fact, the maneuverability made possible by slotted wings might have helped birds compete with pterosaurs and ultimately survive the end-Cretaceous extinction.
T he pennaceous feathers we associate with flight aren’t the only type of feather birds possess. Feathers in different regions of the body vary in size, shape and function. You can think of feather form as a spectrum, with the large, relatively stiff flight feathers of the wing and tail at one end and the short, fluffy down feathers that sit close to the body at the other. All of them have a central shaft and softer “barbs” that branch out from the shaft. In flight feathers, the barbs interlock like Velcro teeth to form the smooth, windproof surface of the vanes. In down feathers, the barbs are loosely structured and fluffy to trap heat. Many of the other kinds of feathers combine aspects of these two types. The contour feathers that streamline a bird’s body, for example, have vaned tips like flight feathers and noninterlocking barbs like down ones. The bristle feathers that typically occur on the face and may serve protective and sensory purposes meld the flight feathers’ stiff shafts (called rachises) with the down feathers’ fluffy base.

The wing of the Greater Prairie-Chicken, a type of grouse, has a slotted tip that helps the bird burst into flight when startled.
Robert Clark
In recent years researchers have begun to piece together the intricate process by which feathers develop. Like scales, spines and hairs, feathers are skin appendages. Scientists have known for a while that they arise from structures in the skin. But how can an animal produce feathers with different anatomies across its body?
My colleagues and I, led by Cheng-Ming Chuong of the University of Southern California, related the developmental biology of various kinds of pennaceous feathers to their mechanical properties. These feathers begin as a tube that essentially unzips along its length, forming the two vanes. Several genes and molecules interact with one another and with the environment to determine the amount of interlocking in the barbs that make up the vanes, the size and shape of the rachises, and whether the shaft is filled with a “foam” that makes it stiff relative to its weight. We found that different feather types have varying specializations in their overall stiffness, their tendency to twist, and the distribution of the foam in the shaft. These variations depend to some extent on the work of different genes, but most of the differentiation is the result of changes in how the genes are regulated—that is, when they are turned on or off or how active they are during feather development.
Scientists have also shown a recent surge of interest in another category of feathers: display feathers, the showy feathers that help to attract mates. Display feathers may dazzle an observer with their colors (think of a hummingbird’s glittering throat), or they may attain eye-catching proportions, like the feathers that make up a peacock’s crest and train. The conventional wisdom about display feathers holds that they are strictly products of sexual selection, in which mate choice drives the evolution of a trait. These days, however, researchers around the globe, me included, are coming to see display feathers not as exclusively sexually selected traits with no mechanical properties of interest but instead as complex compromises between the pressures of social biology and mechanobiology.
To wit: long display feathers don’t grow just anywhere on the body. They most often occur on the lower back and tail, where they interfere comparatively little with flight performance. Take, for example, the Resplendent Quetzal, a small, colorful bird native to the cloud forests of Mexico and Central America with tail feathers that can grow up to three feet long on males during the breeding season. The tail streamers might not be shaped solely by sexual selection. Evidence indicates that the streamers of some birds produce at least a little aerodynamic force, enough to support much of their added weight. The quetzal’s streamers, for their part, lost their tight interlocking structure, making the vanes a pennaceous-downy hybrid that lets much of the airflow pass through without producing much lift. This arrangement is most likely an adaptation to prevent these feathers from being highly destabilizing. These flashy feathers still increase the cost of flying because they add drag, but that cost may well be less than has been assumed.

The Barn Owl‘s primary feathers have features that allow this bird of prey to fly silently.
The microstructure of display feathers, especially tail streamers, may also be more finely tuned than previously thought. Feather structure provides a balance of stiffness, weight and shape. The feathers must hold their shape well enough, even at extreme lengths, to be effective signals. But they cannot be so stiff as to destabilize the bird during gusty winds or tight maneuvers. There’s a particular range of flexibility that shows off the feather to best effect while minimizing detrimental impacts on flight performance.
O ne of the aspects of feathers that has long fascinated me is their adaptability. Under varying conditions and evolutionary pressures, they can become specialized for everything from speed and maneuverability to insulation or display. Some of the most fascinating adaptations can be found in owls.
Facial disks are an especially conspicuous feature of owls . These broad, semicircular fans of feathers around the eyes and ears give owls their distinctive appearance. The skull of an owl is actually quite long and narrow, but the feathers enveloping it completely change the contours of the animal. These facial disks are not just for looks. They do a remarkably good job of funneling sound to the owl’s ears. The disks, along with vertically offset ears and exceptionally sensitive middle and inner ear structures, make owls so good at determining the origin of a sound that they can zero in on prey without seeing it at all (they still use vision to make the final capture, though).
I have worked with quite a few owls over the years, particularly individuals being rehabilitated after injury. One such owl couldn’t be released because a car strike had left him completely blind. Yet if someone tossed food onto one of his perches, the gentle thud of it landing was enough for him to pounce on it perfectly. (Readers may also find solace in knowing that he still flew, having memorized his enclosure, and was regularly taken around for walks and neck scratches.)
Still, that exceptional sense of hearing wouldn’t get owls very far without some additional feather adaptations. Other nocturnal creatures can also hear very well, and an owl whose feathers were rustling in flight would be hard-pressed to get close to its vigilant prey. Furthermore, owls might not hear quietly creeping prey if their own feather sounds covered the faint noises of their targets. Owls solved both problems by evolving feather traits that make them inaudible during flight.

The extremely stiff feathers of hummingbirds such as Anna’s Hummingbird help to support their distinctive, hovering flight.
Kathleen Reeder Wildlife Photography/Getty Images
It is hard to appreciate just how quiet owls are. Even ultrasensitive microphones, if properly calibrated, aimed exactly right and set to maximum sensitivity in a silent space, can just barely pick up sounds from a flying owl ... sometimes. For all practical purposes, owls are silent. They are so eerily noiseless that even if they fly over your head close enough for you to feel their wake, you will still hear absolutely nothing. In a dark space, they are essentially undetectable. All the owl wing sounds you hear in the Harry Potter movies and other films? Those are added in.
Owls achieve this stealth with a few different feather adaptations. To start, their feathers have a “velvety” surface that silences them when they move against one another. More important, the feathers on the leading edge of an owl’s wing have a set of comblike structures, whereas those on the trailing edge have fluffy fringes. The leading-edge comb stirs the air in a specific way called micro vorticity. These tiny, swirling streams of air cause the main flow to stick to the wing. In aerodynamic speak, we say the combs “inject vorticity into the boundary layer.” When this modified flow then passes through the trailing-edge fringes, the net result is a wake that contains no coherent waves of linear pressure and therefore no sound. Put another way, there are no vibrations from the interactions between feathers and the air capable of producing sound.
These specializations have deep roots. Modern-day owls belong to one of two groups: the tytonids (represented by Barn Owls and Bay Owls) and the strigids (all other living owls). Their last common ancestor existed at least 50 million years ago. Because owls in both groups exhibit silent flight, this trait probably dates to their common ancestor. In other words, owls have been surreptitiously coursing the night skies for more than 50 million years.
Not surprisingly, some of the most extreme feather adaptations are found in birds with the most extreme ecological specializations. One way feathers can adapt to a particular way of life is by increasing or decreasing in stiffness. Coincidentally, the stiffest feathers are found in two groups of birds that are otherwise as different as can be: hummingbirds and penguins .
Hummingbirds have ultrastiff feathers as an adaptation to the exceptionally high flapping frequencies and unusual flapping stroke they use to hover in front of flowers while sipping nectar. Unlike most birds, hummingbirds can get a substantial amount of weight support and thrust from their upstroke, not just their downstroke. They do this by rotating their shoulder to flip the wing over completely. The wing needs to be very stiff for this method to work. Reinforcements in the bones of the hummingbird wing provide some of this rigidity; feathers with extremely firm rachises provide the rest.
The flightless penguins, in contrast, have adapted to life in the water and on land. They possess some of the most specialized plumage of all, having converted their entire body covering into a densely packed mosaic of tiny feathers. These feathers are individually quite stiff, and together they form a textured surface over the wings and body that regulates the boundary layer of water against them while the penguin is swimming. In essence, they use a rough coat of feathers to catch and hold a smooth jacket of water. The net effect is a reduction in drag and therefore a lower energetic cost of swimming. The dense feathers also trap just enough air to provide some insulation without making the penguin buoyant, supplementing the fat layer that helps to keep the bird warm.
In the absence of any constraints posed by flight, penguins jettisoned the more typical feather accoutrements of their ancestors in favor of a novel suit of drag-reducing, minimum-buoyancy feathers. These feathers are a key part of the package of adaptations that have made penguins the undisputed diving champions of the avian world, capable of reaching depths of more than 1,600 feet in search of krill, fish, and other aquatic prey.
Feathers are a fantastic model system for understanding how complex structures evolve and how anatomy and behavior influence each other over time. It’s no wonder that the applied science sector has taken note of feathers’ many brilliant features. Already they have led to successful technological innovations. The Velcro-like mechanism that connects the barbs of pennaceous feathers is the basis for an advanced temporary fastening system. The silencing fringes of owl feathers have inspired ventilation-quieting systems. The surface texture and boundary-layer-control principles of penguin feathers have made their way into robotics, mostly in prototypes.
No doubt feathers will give rise to more clever inventions in the future. We have only to let our creativity take flight.

Long display feathers may be present on the wings, as happens in the Standard-winged Nightjar ( left ) and Pennant-winged Nightjar ( right center ). But they usually grow on the lower back and tail, which minimizes any negative impact on flight, as in the Stephanie’s Astrapia ( left center ) and Resplendent Quetzal ( right ).
Michael B. Habib is a paleontologist and biomechanist at the Natural History Museum of Los Angeles and the University of California, Los Angeles. He studies the anatomy and motion of pterosaurs, birds and feathered dinosaurs.


Exploring The Evolution of Birds: From Dinosaurs to Today
Birds are some of the most fascinating and diverse creatures on our planet, with over 11,000 species scattered across the globe. But did you know that birds are actually descendants of dinosaurs? That’s right – the evolution of birds is a story that spans over 150 million years, from the earliest feathered dinosaurs to the modern avian species we see today.
Key Takeaways:
The evolution of birds.
The evolution of birds is a fascinating subject that has captured the attention of scientists and bird enthusiasts alike. Birds are believed to have evolved from dinosaurs, specifically theropods, about 150 million years ago during the Jurassic period.
As birds continued to evolve, they diversified into a wide range of species with different shapes, sizes, and behaviors. Today, there are more than 11,000 species of birds , ranging from tiny hummingbirds to large ostriches.
The Origins of Birds: From Dinosaurs to Avian Ancestors
The evolution of birds can be traced back to their distant dinosaur ancestors . The fossil records have given us a glimpse into the transition from feathered dinosaurs to avian ancestors. This section will discuss the evidence of bird ancestors and feathered dinosaurs that led to the emergence of bird-like features.
Other important bird-like dinosaurs include the Microraptor , which had feathers on all four of its limbs, and the Anchiornis , which had a mix of bird and dinosaur characteristics in its feathers and skeleton.
The Early Evolution of Birds: From Archaeopteryx to Enantiornithines
The early evolution of birds is a topic of great interest to paleontologists, as it sheds light on the development of modern bird species. Fossil records have revealed several key species that contributed to the diversification of birds.
Archaeopteryx
Enantiornithines.
Fossil records of these and other early bird species provide an important window into the transition from dinosaurs to modern birds. They also demonstrate how early bird species began to diversify and adapt to their environments, paving the way for the many different bird species we see today.
The Diversification of Birds: Adaptive Radiation and Speciation
The timeline of bird evolution can be traced through the fossil record, which shows a progression from early bird-like dinosaurs to the emergence of distinct bird species. Notable examples of early birds include Archaeopteryx, which had feathers and wings but also retained some dinosaur-like features such as sharp teeth.
The Impact of Adaptive Radiation on Bird Species Development
Adaptive radiation also played a key role in the speciation of birds, where different populations of a species become genetically isolated and evolve into distinct species. This process can occur when populations are separated by physical barriers such as mountains or bodies of water, or by differences in behavior or mating preferences.
Understanding Bird Phylogeny: Tracing the Genetic Tree
The phylogenetic relationships among birds are complex and constantly evolving as new species are discovered and genetic analyses become more sophisticated. Birds are classified into several major groups, including the Paleognathae (ostriches, emus, and kiwis) and the Neognathae (all other birds).
The Branches of the Bird Family Tree
The bird family tree is divided into many branches, each representing a different evolutionary lineage. Some of the major branches of the bird family tree include:
The Significance of Bird Phylogeny
Understanding bird phylogeny is essential for studying the evolution of birds and their adaptations. By tracing the genetic tree, scientists can identify common ancestors and determine how different bird species are related to one another. This knowledge can also help inform conservation efforts by identifying which species are most closely related and which are most at risk of extinction.
The Remarkable Adaptations of Birds: From Flight to Beak Diversity
Flight is perhaps the most iconic adaptation associated with birds. While not all birds can fly, those that can have developed a range of features that make powered flight possible. The most obvious of these is the presence of wings, which are modified forelimbs that allow birds to generate lift and achieve sustained flight.
Hummingbirds, on the other hand, have long, slender beaks that allow them to sip nectar from flowers. Birds of prey have developed hooked beaks that enable them to tear apart their prey, while finches have developed short, conical beaks that are ideal for cracking seeds and nuts. The diversity of beak shapes and sizes in birds is a testament to the adaptability of this group of animals.
The evolution of these adaptations has occurred over millions of years, and has been shaped by a range of factors including changes in climate, habitat availability, and competition with other animals.
The Role of Environment in Bird Species Development
For example, the development of powerful chest muscles in birds is thought to have been influenced by the need to generate lift in high altitude environments. Similarly, the evolution of specialized beaks in birds has been influenced by the availability of different food sources.
Human Impact on Bird Evolution: From Extinctions to Conservation
Bird species have been evolving for millions of years, but human activities have had a significant impact on that evolution. Many bird species have become extinct due to habitat destruction, introduced species, hunting, and climate change.
Other bird species that have gone extinct due to human activities include the great auk and the passenger pigeon. Today, many bird species are facing declining population numbers, with some on the verge of extinction.
| Human Activities Impacting Bird Evolution | Examples |
|---|---|
| Habitat Destruction | Deforestation, urbanization |
| Introduced Species | Invasive predators, competitors for resources |
| Hunting | Overharvesting, illegal trade |
| Climate Change | Altered migration patterns, habitat loss |
As humans continue to impact the environment and drive changes in bird populations, it is important to track the ongoing evolution of bird species. By doing so, researchers can identify the factors that are driving species changes and develop more effective conservation strategies to protect them.
The Future of Bird Evolution: Adaptation in a Changing World
Another area where we may see evolution in bird species is in response to pollution. The impact of pollution on birds is complex, with some species appearing to be adapting to survive in highly polluted environments.
Additionally, rising sea levels may impact shorebird populations, potentially leading to changes in migration patterns or breeding locales.
The Evolution of Birds: A Significant and Ongoing Story
The evolution of birds is a fascinating story that began over 150 million years ago. Birds have evolved from dinosaurs and have developed into distinct species with a wide array of adaptations and behaviors. The study of avian evolution has provided significant insights into the history of life on Earth and continues to be an exciting area of research and discovery.
Significance of Avian Evolution
The ongoing story of avian evolution.
The research on avian evolution is ongoing, and new discoveries are being made regularly. Scientists continue to study the relationships between different bird groups and the development of new adaptations. Understanding the evolution of birds can also help us develop strategies to protect endangered species and conserve their habitats.
FAQs: Exploring The Evolution of Birds
What is the connection between birds and dinosaurs, are there any fossil records that show the transition from dinosaurs to birds.
Yes, there have been several fossil discoveries that provide evidence of the transition from dinosaurs to birds. One of the most famous examples is Archaeopteryx, a species that had both bird-like and dinosaur-like features.
How did birds evolve different physical characteristics over time?
How do scientists classify and understand the evolutionary history of birds, what are some notable adaptations of birds.
Birds have remarkable adaptations, such as the ability to fly and the evolution of various beak shapes and sizes. These adaptations have allowed birds to thrive in diverse environments and specialize in different feeding strategies.
How has human activity affected bird evolution?
What does the future hold for bird evolution.
https://worldanimalfoundation.org/advocate/how-many-animals-are-in-the-world/
Meet Vince, the passionate founder and author of Learn Bird Watching, boasting 30 years of birding experience. With an unwavering mission to empower fellow bird enthusiasts, Vince shares invaluable wisdom and guidance. As a dedicated moderator and contributor to Quora's Bird Watchers' Club, he actively engages with the birding community, where his insightful answers have garnered over 571,082 views and over 2,725 upvotes. Whether you're a budding birder or a seasoned avian aficionado, his wealth of knowledge is at your service.
Accessibility Bar
- Share full article
Advertisement
Supported by
The Big City Is Vibrant. Birds There Might Be Getting Less So.
Recent studies show that certain feather pigments can help neutralize toxic pollution. It means darker, duller birds could have a survival advantage.

By Marta Zaraska
Some popular city dwellers appear to be losing their colorful allure, and not just the dirty birds.
According to a study published this summer in the journal Landscape and Planning that looked at 547 bird species in China, birds that live in cities are duller and darker on average than their rural counterparts. A similar conclusion emerged from an analysis of 59 studies published in March in Biological Reviews : Urban feathers are not as bright, with yellow, orange and red feathers affected most.
Often, city birds are covered in grime. But even if you could give them all a good bird bath, chances are their brightness still wouldn’t match that of their country cousins. That’s because of the way pollution, and heavy metals in particular, can interact with melanin, a pigment that makes feathers black, brown and gray.
Studies show that melanin can bind to heavy metals like lead. That means toxic chemicals may be more likely to be stored in plumage in darker and duller birds. And that, in turn, can confer a survival advantage.
“The more melanin you accumulate, the better able you are to sequester these harmful compounds in feathers,” said Kevin McGraw, a biologist at Michigan State University who studies the colors of animals to understand the costs, benefits and evolution of visual signals.
Urban pollution affects avian colors in other ways, too. Research shows that, compared with rural plants, city trees store fewer natural pigments called carotenoids. And pollution is the likely reason. Carotenoids are produced by plants, algae and fungi. They’re what makes red peppers red and carrots orange.
When leaves are low on these pigments, the effects go up the food chain: Leaf-munching caterpillars become deficient in carotenoids, and so do caterpillar-munching birds.
We are having trouble retrieving the article content.
Please enable JavaScript in your browser settings.
Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.
Thank you for your patience while we verify access.
Already a subscriber? Log in .
Want all of The Times? Subscribe .

The Top 10 Largest Birds of Prey

Written by Rebecca Bales
Published: September 17, 2024

How to Add Us to Google News

Advertisement
A bird of prey or a raptor is a bird that hunts and eats other animals. Because the prey is often large relative to the predator, these birds are equipped with sharp, hooked bills to tear into their food and large, sharp talons to grab and hold on to it tightly.
Though people know that eagles and hawks are birds of prey, some are surprised to learn that vultures and condors are also birds of prey. It’s true that vultures and condors are mostly scavengers, but they will hunt for food, especially if the prey is sick or weak. Here is a list of the biggest birds of prey in ascending order.

#10 Steller’s Sea Eagle
This huge eagle from the northeastern part of Asia has a bright yellow beak, yellow talons, dark brown feathers with white bands on its wings, white feathers over the thighs and legs, and a white tail. It is considered the heaviest eagle in the world and usually weighs about 11 to 20 pounds. Its talons are formidable, and the feet also possess spicules at the bottom which let the bird hold onto struggling fish .
The fish the Steller’s sea eagle prefers are types of salmon , stickleback, trout, cod , and pollock. The eagle is powerful enough to take large birds such as geese , swans , and even albatross . The conservation status of Steller’s Sea Eagle is vulnerable .

The Steller’s Sea Eagle is the heaviest eagle in the world, weighing up to 20 pounds.
©GUDKOV ANDREY/Shutterstock.com
#9 White-Tailed Eagle
As the bald eagle is found in America , its close cousin the white-tailed eagle is found largely in Russia and Eurasia . It is a sea eagle that usually lives along bodies of water. It is brown and gray all over save its white tail and can range in length from 26 to 37 inches with a wingspan of between nearly 6 feet to 8 feet.
Females usually weigh from around 9 to 15 pounds while males weigh about 7 to 12 pounds.
White-tailed eagles have the largest population of any eagles in Europe and they are often found along the coast of Norway. They tend to hang out near sea lochs although do roam the areas of islands. This behavior is common among young white-tailed eagles. The species is widely seen in Russia as well as Norway.
The eagle can also be recognized by its calls, which sound like a series of yelps. It often scavenges but is an exceptionally agile flyer and has been seen to snatch fish and waterfowl from bodies of water.

White-Tailed Eagle
is found in Russia and Eurasia.
©Sergey Uryadnikov/Shutterstock.com
#8 Bald Eagle
The national bird of the United States and found throughout much of North America , the bald eagle’s pure white head and neck and white tail and rump are very recognizable. It most often catches live prey, including seabirds such as ducks and seagulls, and fish including salmon . Yet the bald eagle is not above scavenging and even stealing food from other animals, especially the smaller Osprey.
It prefers to roost in tall trees, even though it is challenging for this big bird to fly through a dense canopy. It ranges between 30 and 43 inches long, weighs about 9.47 pounds, and has a wingspan between 6 and 7.5 feet. It takes five years for a bald eagle to achieve its unique plumage, and even then it may still have some of the dark feathers of its younger days.
The bald eagle has been important to humans dating back to ancient Rome when it was used as a symbol for Roman legions who considered it to represent strength.
In 1782, five years after the Declaration of Independence was signed, Charles Thompson, the secretary of Congress, was working on nailing down the design for the official seal of the United States of America. He suggested that a sketch he’d reviewed of a small white eagle be changed to a bald eagle. Since then, the bald eagle has been the national symbol of the USA.

The Bald Eagle is America’s famous national bird.
©Chris Hill/Shutterstock.com
#7 Harpy Eagle
This enormous eagle is renowned for the size of its feet and the fearsomeness of its talons. It is a predator in the rainforests of Central and South America and can be seen flying through the dense trees effortlessly as it hunts possums , snakes , monkeys , and sloths .
Though it’s not the longest eagle, it is one of the largest, with a wingspan of nearly 7 feet and an average length of between 35 and 43 inches. It weighs between 11 and 20 pounds. It’s a striking bird with a slate-colored back, barred, black, and gray tail feathers, light gray head and thighs, and a light-colored breast and belly. The eagle has a crown of feathers that it raises either because it’s threatened or to allow it to hear better or for both reasons. Harpy eagles mate for life and can live between 25 and 35 years in the wild.

The majestic Harpy Eagle can live up to 35 years in the wild.
©MarcusVDT/Shutterstock.com
#6 Philippine Eagle
As its name states, this eagle is found in the Philippines and lives in the rainforest. Because of this, it has a more slender silhouette than most eagles, but it is one of the biggest raptors at around 37 inches long. The bird weighs between 8.9 to 17.6 pounds. It’s brown on top with a white belly and throat, and its wings are large and have round tips. It also has a brown and white crest, yellow legs, powerful talons, and a large, curved beak. The bird’s beak and talons allow it to seize any prey it can handle, from monkeys to rats to birds to small deer . Though this eagle is revered and is the national bird of the Philippines, it’s still critically endangered due to habitat destruction.

The Philippine Eagle has a unique look that helps it blend in with the environment.
©Alaz/Shutterstock.com
#5 Lappet-Faced Vulture
This vulture, also called the Nubian vulture, has two subspecies. One lives in much of Africa while the other is found in the Middle East. It prefers dry, open areas though it can also be seen now and then near human dwellings in search of roadkill. It’s a cousin of the cinereous vulture and is just a little smaller at 37 to 45 inches long with an 8 to 9.5-foot wingspan.
Like other vultures, this one has a bald head, but its skin is wrinkled and folded, which gives the bird its name. The vulture is not only big but aggressive and will often take over a carcass from smaller vultures. This benefits both vultures because the lappet-faced is strong enough to tear open the tough hide of animals such as an elephant or buffalo . In doing this, the lappet-faced vulture and other animals can partake of the meat.

The Lappet-Faced Vulture is known to be aggressive and is famous for its wrinkled skin.
©EcoPrint/Shutterstock.com
#4 Lammergeier
This bird of prey is also called the bearded vulture, but it’s more closely related to eagles and hawks. Indeed, its head is fully feathered, even though it does eat carrion. There are 13 subspecies that are found throughout Africa, Spain , India , Tibet, parts of China , and Siberia. It resides in the mountains, which gives it a good view of prey animals, especially those that have been freshly killed by predators.
These huge birds range in length from 37 to 49 inches and have a wingspan between 7.6 and 9.3 feet. Females are larger. Interestingly, lammergeiers sometimes form threesomes, with two males vying for the same female. They’re also different from other vultures in that they bring pieces of prey to their chicks as opposed to regurgitating them.

The Lammergeier sometimes forms threesomes with two males competing for the same female.
©Petr Salinger/Shutterstock.com
#3 Cinereous Vulture
This vulture is one of the biggest of the Old World vultures. Also called the black vulture or monk vulture, this bird can be almost 4 feet long, weigh around 30 pounds, and have a wingspan that’s a little over 10 feet. It is not only one of the largest of the flying birds but one of the heaviest. It’s found from Spain down into the Middle East and east into China and Mongolia .
Its body is covered with brown feathers, and it has bluish-gray skin. The bird’s head has a fine, dark color, and when it’s flying it can seem completely black to a person on the ground. This illusion gives it one of its names. These vultures, which nest on cliffs, are devoted parents, and their well-fed chicks can weigh more than their parents by the time they fledge.

The Cinereous Vulture is one of the most affectionate parents.
©Karel Bartik/Shutterstock.com
#2 Andean Condor
Though the Andean Condor is about 2 inches shorter than the California condor, it is heavier and has an even longer wingspan. The Andean condor can weigh as much as 33 pounds and have a wingspan from 9 to a little over 10 feet. The female is smaller than the male, which is unusual for raptors. She also differs from him in that he has a comb and a wattle while she doesn’t. Males also have brown eyes while the eyes of females are red.
Like other vultures, they both have naked heads and necks, which makes it less messy to plunge their heads into the innards of rotting carcasses. They can also change the color of the skin of their head and neck according to their mood. This condor is found in the mountains and deserts of western South America .

Andean Condors can change the color of their skin according to their mood.
©BearFotos/Shutterstock.com
#1 California Condor
At between 46 and 55 inches from its head to its tail, the California condor is the largest bird of prey in the Americas. It can weigh as much as 23 pounds and have a nine-and-a-half-foot wingspan. Its head and neck are naked except for some black feathers on the forehead and the ring of black feathers around the neck. This bird of prey was once found up and down the west coast of North America but now is found only in the deserts of south-central California.
California condors, who do mostly eat carrion , are fastidious about their persons and spend much time grooming and preening themselves. A California condor can live as long as 45 years in the wild.

The California Condor can live for as long as 45 years.
©MTKhaled mahmud/Shutterstock.com
Summary of the 10 Largest Birds of Prey
Here’s a review of the birds of prey that make the cut for the largest in the world:
| Rank | Bird of Prey | Average Feet of Wingspan |
|---|---|---|
| 1 | California Condor | 9.5 |
| 2 | Andean Condor | 9 – 10 |
| 3 | Cinereous Vulture | Over 10 |
| 4 | Lammergeier | 7.6 – 9.3 |
| 5 | Lappet-Faced Vulture | 8 – 9.5 |
| 6 | Philippine Eagle | 6 – 7.3 |
| 7 | Harpy Eagle | Almost 7 |
| 8 | Bald Eagle | 6 – 7.5 |
| 9 | White-Tailed Eagle | 6 – 8 |
| 10 | Steller’s Sea Eagle | 6.4 – 8.2 |

Watch the World’s Fastest Bird That Can Dive-Bomb Its Prey at 240 MPH

Watch A Huge Heron Slurp Up A Live Snake Like A Pasta Noodle

Tiny Crab Nearly Drowns a Large Bald Eagle in Impressive Fight
Rebecca Bales
Thank you for reading! Have some feedback for us? Contact the AZ Animals editorial team .
- Sourcing Our Content
- Privacy Policy
- Terms & Conditions
- Editorial Guidelines
As an Amazon Associate I earn from qualifying purchases. Learn more about us & read our affiliate disclosure .
A-Z-Animals.com is Copyright © 2008 - 2024 A-Z Animals

IMAGES
VIDEO
COMMENTS
The origin of birds is now one of the best understood major transitions in the history of life. It has emerged as a model case for using a combination of data from fossils, living species, genealogies, and numerical analyses to study how entirely new body plans and behaviors originate, and how prominent living groups achieved their diversity over hundreds of millions of years of evolution 2, 3.
Abstract. Birds are one of the most recognizable and diverse groups of modern vertebrates. Over the past two decades, a wealth of new fossil discoveries and phylogenetic and macroevolutionary studies has transformed our understanding of how birds originated and became so successful. Birds evolved from theropod dinosaurs during the Jurassic ...
Although well studied, the evolutionary relationships among major avian groups are contentious (1-6).Recovering deep evolutionary relationships in birds is difficult, probably reflecting a rapid divergence early in their evolutionary history (1-3, 7, 8) that has resulted in many distinctive, morphologically cohesive groups (e.g., owls, parrots, and doves) with few, if any, extant ...
Bird clades originating near the K-Pg boundary exhibited numerous shifts in the mode of molecular evolution, suggesting a burst of genomic heterogeneity at this point in Earth's history. These inferred shifts in substitution patterns were closely related to evolutionary shifts in developmental mode, adult body mass, and patterns of ...
Further information on research design is available in the ... Claramunt, S. & Cracraft, J. A new time tree reveals Earth history's imprint on the evolution of modern birds.
The discovery that birds evolved from small carnivorous dinosaurs of the Late Jurassic was made possible by recently discovered fossils from China, South America, and other countries, as well as by looking at old museum specimens from new perspectives and with new methods. The hunt for the ancestors of living birds began with a specimen of Archaeopteryx, the first known bird, discovered in the ...
Birds evolved from theropod dinosaurs during the Jurassic (around 165-150 million years ago) and their classic small, lightweight, feathered, and winged body plan was pieced together gradually over tens of millions of years of evolution rather than in one burst of innovation. Early birds diversified throughout the Jurassic and Cretaceous ...
A new time tree for birds. We identified avian clades that have a relatively old and well-characterized fossil whose affinities are well established by phylogenetic analysis and/or derived morphologies. These fossils set a minimum bound for the stem age of the corresponding clade and its sister group (Fig. 1A) (31 - 33).
There is strong evidence for the diversification of stem birds immediately before the K-Pg boundary (13, 34 - 36), as well as the subsequent radiation of crown birds in the earliest Paleogene (12, 34 - 36), as revealed by our DRTT and morphological evolution rate analyses (Figs. 1 and 2).
The research revealed that most modern bird groups appeared within a very small evolutionary window of only five million years. These findings support the hypothesis that birds made the most of opportunities after an asteroid struck earth 66 million years ago wiping out the dinosaurs. The comprehensive study was led by Assistant Professor ...
The research is part of the Bird 10,000 Genomes (B10K) Consortium, which aims to sequence the genomes of every single species of bird.The study was led by Zhang together with Assistant Professor Josefin Stiller from the University of Copenhagen and Associate Professor Siavash Mirarab from the University of California, San Diego, and has been published in the journal Nature.
MORE TO EXPLORE. Gradual Assembly of Avian Body Plan Culminated in Rapid Rates of Evolution across the Dinosaur-Bird Transition. Stephen L. Brusatte et al. in Current Biology, Vol. 24, No. 20 ...
Scientists have tended to view modern bird diversity as the result of a burst of evolutionary activity that occurred after the fateful day 66 million years ago when a six-mile-wide asteroid struck ...
New research suggests that bird ancestors shrank fast, indicating that the diminutive size was an important and advantageous trait, quite possibly an essential component in bird evolution.
The ancestors of birds were bipedal, terrestrial, agile, cursorial and carnivorous or omnivorous. Apart from a perching foot and some skeletal fusions, a great many characters that are usually considered 'avian' (e.g. the furcula, the elongated forearm, the laterally flexing wrist and apparently feathers) evolved in non-avian theropods for ...
Research on the origin and evolution of birds has gathered pace in recent years, aided by a continuous stream of new fossil finds as well as molecular phylogenies. Bird origins, in particular, are now better understood than those of mammals, for which the early fossil record is relatively poor compared with that of birds. ...
Abstract. Birds evolved from dinosaurs, but how long did this evolutionary transition take? Twenty years ago, it was widely assumed that the first bird— Archaeopteryx , which lived in the Late ...
Additionally, avian evolution research has previously relied on smaller gene sets, which caused data inconsistencies, further inhibiting any consensus on what the tree looked like. For this body of work, the researchers changed the game by using full genome mapping of 48 species, which together encompass all the bird lineages alive today.
A turning point came in the early twentieth century with the writings of Gerhard Heilmann of Denmark.An artist by trade, Heilmann had a scholarly interest in birds and from 1913 to 1916, expanding on earlier work by Othenio Abel, [12] published the results of his research in several parts, dealing with the anatomy, embryology, behavior, paleontology, and evolution of birds. [13]
If, instead, you go to Australia,... Since 1970, bird populations in North America have declined by 29%, a net loss of 3 billion birds (figure 12.1). At least 40% of the world's species of birds are declining, and 1,469—1 in every 8 species—are threatened with complete extinction.¹ Birds are not alone in peril.
The wing of the Greater Prairie-Chicken, a type of grouse, has a slotted tip that helps the bird burst into flight when startled. Robert Clark. In recent years researchers have begun to piece ...
Ongoing research into bird evolution will continue to shed light on the ways in which our actions are shaping the natural world around us. The Evolution of Birds: A Significant and Ongoing Story. The evolution of birds is a fascinating story that began over 150 million years ago. Birds have evolved from dinosaurs and have developed into ...
Research shows that, compared with rural plants, city trees store fewer natural pigments called carotenoids. And pollution is the likely reason. Carotenoids are produced by plants, algae and fungi.
Birds evolved from dinosaurs, but how long did this evolutionary transition take? Twenty years ago, it was widely assumed that the first bird—Archaeopteryx, which lived in the Late Jurassic (see the photo)—evolved its feathers, wings, and ability to fly within just 10 million years or so.Since then, it has become clear that most of the 30 or more characteristics that distinguished the ...
It also has a brown and white crest, yellow legs, powerful talons, and a large, curved beak. The bird's beak and talons allow it to seize any prey it can handle, from monkeys to rats to birds to small deer. Though this eagle is revered and is the national bird of the Philippines, it's still critically endangered due to habitat destruction.
We found evidence for dissemination of HPAI viruses between wild bird species, wild birds and domestic poultry, as well as wild birds and wild mammals. Continued monitoring for and genomic characterization of HPAI viruses in Alaska can improve our understanding of the evolution and dispersal of these economically costly and ecologically ...