If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

Unit 6: Thermodynamics
About this unit.
This unit examines the role of energy in physical and chemical processes. Learn about heat transfer, calorimetry, enthalpy of reaction, Hess’s law, and more. Practice what you’ve learned and study for the AP Chemistry exam with more than 55 AP-aligned questions.
Endothermic and exothermic processes
- Endothermic and exothermic processes (Opens a modal)
- Representing endothermic and exothermic processes using energy diagrams (Opens a modal)
Heat transfer and thermal equilibrium
- Heat transfer and thermal equilibrium (Opens a modal)
Heat capacity and calorimetry
- Heat capacity (Opens a modal)
- Constant-pressure calorimetry (Opens a modal)
- Worked example: Measuring the energy content of foods using soda-can calorimetry (Opens a modal)
- Constant-volume calorimetry (Opens a modal)
- Heat capacity and calorimetry Get 3 of 4 questions to level up!
Energy of phase changes
- Enthalpy and phase changes (Opens a modal)
- Heating curve for water (Opens a modal)
Introduction to enthalpy of reaction
- Enthalpy of reaction (Opens a modal)
- Worked example: Measuring enthalpy of reaction using coffee-cup calorimetry (Opens a modal)
- Introduction to enthalpy of reaction Get 3 of 4 questions to level up!
Hess's law
- Hess's law (Opens a modal)
- Worked example: Using Hess's law to calculate enthalpy of reaction (Opens a modal)
- Hess's law Get 3 of 4 questions to level up!
Enthalpy of formation
- Enthalpy of formation (Opens a modal)
- Enthalpy of formation Get 3 of 4 questions to level up!
Bond enthalpies
- Bond enthalpies (Opens a modal)
- Worked example: Using bond enthalpies to calculate enthalpy of reaction (Opens a modal)
- Bond enthalpies Get 3 of 4 questions to level up!

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction & Top Questions
Thermodynamic states
- Thermodynamic equilibrium
- Temperature
- Work and energy
- Total internal energy
- Heat engines
- Isothermal and adiabatic processes
- The second law of thermodynamics
- Entropy and efficiency limits
- Entropy and heat death
- Entropy and the arrow of time
- Thermodynamic potentials
- Gibbs free energy and chemical reactions
- Enthalpy and the heat of reaction
- Work of expansion and contraction
- Equations of state
- Heat capacity and specific heat
- Heat capacity and internal energy
- Entropy as an exact differential
- The Clausius-Clapeyron equation
- Concluding remarks

thermodynamics
Our editors will review what you’ve submitted and determine whether to revise the article.
- UEN Digital Press with Pressbooks - Introductory Chemistry - Introduction to Thermodynamics
- Khan Academy - Thermodynamics article
- Lehman College - Thermodynamics
- ACS Publications - A View on the Future of Applied Thermodynamics
- Stanford University - Thermodynamics
- NASA - What Is Thermodynamics?
- Live Science - What is thermodynamics?
- National Library of Medicine - A History of Thermodynamics: The Missing Manual
- Chemistry LibreTexts - Thermodynamics
- Table Of Contents

What is thermodynamics?
Thermodynamics is the study of the relations between heat, work, temperature, and energy. The laws of thermodynamics describe how the energy in a system changes and whether the system can perform useful work on its surroundings.
Is thermodynamics physics?
Yes, thermodynamics is a branch of physics that studies how energy changes in a system. The key insight of thermodynamics is that heat is a form of energy that corresponds to mechanical work (that is, exerting a force on an object over a distance).
thermodynamics , science of the relationship between heat , work , temperature , and energy . In broad terms, thermodynamics deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work .

Heat was not formally recognized as a form of energy until about 1798, when Count Rumford ( Sir Benjamin Thompson ), a British military engineer, noticed that limitless amounts of heat could be generated in the boring of cannon barrels and that the amount of heat generated is proportional to the work done in turning a blunt boring tool. Rumford’s observation of the proportionality between heat generated and work done lies at the foundation of thermodynamics. Another pioneer was the French military engineer Sadi Carnot , who introduced the concept of the heat-engine cycle and the principle of reversibility in 1824. Carnot’s work concerned the limitations on the maximum amount of work that can be obtained from a steam engine operating with a high-temperature heat transfer as its driving force. Later that century, these ideas were developed by Rudolf Clausius , a German mathematician and physicist, into the first and second laws of thermodynamics, respectively.
The most important laws of thermodynamics are:
- The zeroth law of thermodynamics. When two systems are each in thermal equilibrium with a third system, the first two systems are in thermal equilibrium with each other. This property makes it meaningful to use thermometers as the “third system” and to define a temperature scale.
- The first law of thermodynamics , or the law of conservation of energy. The change in a system’s internal energy is equal to the difference between heat added to the system from its surroundings and work done by the system on its surroundings. In other words, energy can not be created or destroyed but merely converted from one form to another.
- The second law of thermodynamics . Heat does not flow spontaneously from a colder region to a hotter region, or, equivalently, heat at a given temperature cannot be converted entirely into work. Consequently, the entropy of a closed system, or heat energy per unit temperature, increases over time toward some maximum value. Thus, all closed systems tend toward an equilibrium state in which entropy is at a maximum and no energy is available to do useful work.
- The third law of thermodynamics . The entropy of a perfect crystal of an element in its most stable form tends to zero as the temperature approaches absolute zero . This allows an absolute scale for entropy to be established that, from a statistical point of view, determines the degree of randomness or disorder in a system.
Although thermodynamics developed rapidly during the 19th century in response to the need to optimize the performance of steam engines, the sweeping generality of the laws of thermodynamics makes them applicable to all physical and biological systems. In particular, the laws of thermodynamics give a complete description of all changes in the energy state of any system and its ability to perform useful work on its surroundings.
This article covers classical thermodynamics, which does not involve the consideration of individual atoms or molecules . Such concerns are the focus of the branch of thermodynamics known as statistical thermodynamics, or statistical mechanics , which expresses macroscopic thermodynamic properties in terms of the behaviour of individual particles and their interactions. It has its roots in the latter part of the 19th century, when atomic and molecular theories of matter began to be generally accepted.
Fundamental concepts
The application of thermodynamic principles begins by defining a system that is in some sense distinct from its surroundings. For example, the system could be a sample of gas inside a cylinder with a movable piston , an entire steam engine , a marathon runner, the planet Earth , a neutron star , a black hole , or even the entire universe . In general, systems are free to exchange heat , work , and other forms of energy with their surroundings.
A system’s condition at any given time is called its thermodynamic state. For a gas in a cylinder with a movable piston , the state of the system is identified by the temperature , pressure , and volume of the gas. These properties are characteristic parameters that have definite values at each state and are independent of the way in which the system arrived at that state. In other words, any change in value of a property depends only on the initial and final states of the system, not on the path followed by the system from one state to another. Such properties are called state functions. In contrast, the work done as the piston moves and the gas expands and the heat the gas absorbs from its surroundings depend on the detailed way in which the expansion occurs.
The behaviour of a complex thermodynamic system, such as Earth’s atmosphere , can be understood by first applying the principles of states and properties to its component parts—in this case, water , water vapour, and the various gases making up the atmosphere. By isolating samples of material whose states and properties can be controlled and manipulated , properties and their interrelations can be studied as the system changes from state to state.
Thermodynamics
Thermodynamics deals with the concepts of heat and temperature and the inter-conversion of heat and other forms of energy. The four laws of thermodynamics govern the behaviour of these quantities and provide a quantitative description. William Thomson, in 1749, coined the term thermodynamics.
|
|
What is Thermodynamics?
Thermodynamics in physics is a branch that deals with heat, work and temperature, and their relation to energy, radiation and physical properties of matter.

The video is a rapid revision of thermodynamics for JEE Main, presented by Rakhi Ma’am through short notes and previous year questions (PYQs).

Distinction Between Mechanics and Thermodynamics
The distinction between mechanics and thermodynamics is worth noting. In mechanics, we solely concentrate on the motion of particles or bodies under the action of forces and torques. On the other hand, thermodynamics is not concerned with the motion of the system as a whole. It is only concerned with the internal macroscopic state of the body.
Thermodynamics Timeline

Different Branches of Thermodynamics
Thermodynamics is classified into the following four branches:
Classical Thermodynamics
Statistical thermodynamics, chemical thermodynamics, equilibrium thermodynamics.
In classical thermodynamics, the behaviour of matter is analysed with a macroscopic approach. Units such as temperature and pressure are taken into consideration, which helps the individuals calculate other properties and predict the characteristics of the matter undergoing the process.
In statistical thermodynamics, every molecule is under the spotlight, i.e. the properties of every molecule and how they interact are taken into consideration to characterise the behaviour of a group of molecules.
Chemical thermodynamics is the study of how work and heat relate to each other in chemical reactions and in changes of states.
Equilibrium thermodynamics is the study of transformations of energy and matter as they approach the state of equilibrium.
Basic Concepts of Thermodynamics – Thermodynamic Terms
Thermodynamics has its own unique vocabulary associated with it. A good understanding of the basic concepts forms a sound understanding of various topics discussed in thermodynamics preventing possible misunderstandings.
Thermodynamic Systems

A thermodynamic system is a specific portion of matter with a definite boundary on which our attention is focused. The system boundary may be real or imaginary, fixed or deformable. There are three types of systems:
- Isolated System – An isolated system cannot exchange energy and mass with its surroundings. The universe is considered an isolated system.
- Closed System – Across the boundary of the closed system, the transfer of energy takes place but the transfer of mass doesn’t take place. Refrigerator, compression of gas in the piston-cylinder assembly are examples of closed systems.
- Open System – In an open system, the mass and energy both may be transferred between the system and surroundings. A steam turbine is an example of an open system.
| ☓ | ☓ | ☓ | |
| ✓ | ✓ | ✓ | |
| ☓ | ✓ | ✓ | |
Surrounding
Everything outside the system that has a direct influence on the behaviour of the system is known as a surrounding.
Thermodynamic Process
A system undergoes a thermodynamic process when there is some energetic change within the system that is associated with changes in pressure, volume and internal energy.
There are four types of thermodynamic processes that have their unique properties, and they are:
- Adiabatic Process – A process where no heat transfer into or out of the system occurs.
- Isochoric Process – A process where no change in volume occurs and the system does no work.
- Isobaric Process – A process in which no change in pressure occurs.
- Isothermal Process – A process in which no change in temperature occurs.
Read More: Thermodynamic Process
A thermodynamic cycle is a process or a combination of processes conducted such that the initial and final states of the system are the same. A thermodynamic cycle is also known as cyclic operation or cyclic processes.
Thermodynamic Equilibrium
At a given state, all properties of a system have fixed values. Thus, if the value of even one property changes, the system’s state changes to a different one. In a system that is in equilibrium, no changes in the value of properties occur when it is isolated from its surroundings.
- When the temperature is the same throughout the entire system, we consider the system to be in thermal equilibrium .
- When there is no change in pressure at any point of the system, we consider the system to be in mechanical equilibrium .
- When the chemical composition of a system does not vary with time, we consider the system to be in chemical equilibrium .
- Phase equilibrium in a two-phase system is when the mass of each phase reaches an equilibrium level.
A thermodynamic system is said to be in thermodynamic equilibrium if it is in chemical equilibrium, mechanical equilibrium and thermal equilibrium and the relevant parameters cease to vary with time.
You may also want to check out these topics given below!
- Kelvin Planck Statement
- Darcy Weisbach Equation Derivation
- Kinetic Theory Of Gases Derivation
- Relation Between Kp And Kc
Thermodynamic Properties
Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system’s state. Thermodynamic properties may be extensive or intensive .
- Intensive properties are properties that do not depend on the quantity of matter. Pressure and temperature are intensive properties.
- In the case of extensive properties, their values depends on the mass of the system. Volume, energy, and enthalpy are extensive properties.
What is Enthalpy?
Enthalpy is the measurement of energy in a thermodynamic system. The quantity of enthalpy equals the total heat content of a system, equivalent to the system’s internal energy plus the product of volume and pressure.
Mathematically, the enthalpy, H, equals the sum of the internal energy, E, and the product of the pressure, P, and volume, V, of the system.
What is Entropy?
Entropy is a thermodynamic quantity whose value depends on the physical state or condition of a system. In other words, it is a thermodynamic function used to measure the randomness or disorder.
For example, the entropy of a solid, where the particles are not free to move, is less than the entropy of a gas, where the particles will fill the container.
Thermodynamic Potentials
Thermodynamic potentials are quantitative measures of the stored energy in a system. Potentials measure the energy changes in a system as they evolve from the initial state to the final state. Different potentials are used based on the system constraints, such as temperature and pressure.
Different forms of thermodynamic potentials along with their formula are tabulated below:
Thermodynamics Solved Problems
Calculate ΔG at 290 K for the following reaction: \(\begin{array}{l}2NO_{(g)} + O_{2(g)} + 2NO_{2(g)} \end{array} \)
ΔH = -120kJ and ΔS = -150JK -1
To make the unit of ΔS the same as ΔH, we have to convert the unit of ΔS as follows:
We know that,
Therefore, ΔG is -77kJ.
Watch the video to know the top seven JEE Thermodynamics questions.

Laws of Thermodynamics
Thermodynamics laws define the fundamental physical quantities like energy, temperature and entropy that characterize thermodynamic systems at thermal equilibrium. These thermodynamics laws represent how these quantities behave under various circumstances.
How many laws of thermodynamics are there?
There are four laws of thermodynamics and are given below:
- Zeroth law of thermodynamics
- First law of thermodynamics
- Second law of thermodynamics
- Third law of thermodynamics
In the next few sections, we will discuss each of the laws of thermodynamics in detail.
Zeroth Law of Thermodynamics
The Zeroth law of thermodynamics states that if two bodies are individually in equilibrium with a separate third body, then the first two bodies are also in thermal equilibrium with each other.
This means that if system A is in thermal equilibrium with system C and system B is also in equilibrium with system C, then system A and B are also in thermal equilibrium.
An example demonstrating the Zeroth Law
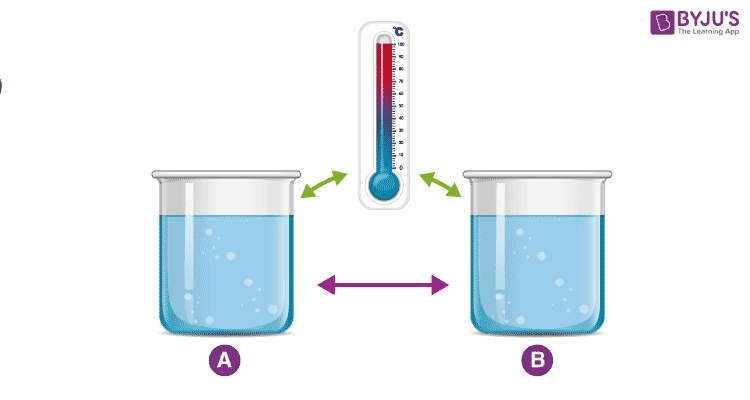
Consider two cups A and B, with boiling water. When a thermometer is placed in cup A, it gets warmed up by the water until it reads 100 °C. When it reads 100 °C, we say that the thermometer is in equilibrium with cup A. When we move the thermometer to cup B to read the temperature, it continues to read 100 °C. The thermometer is also in equilibrium with cup B. By keeping in mind the zeroth law of thermodynamics, we can conclude that cup A and cup B are in equilibrium with each other.
The zeroth law of thermodynamics enables us to use thermometers to compare the temperature of any two objects that we like.
First Law of Thermodynamics
First law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed, but it can be changed from one form to another.
The first law of thermodynamics may seem abstract, but we will get a clearer idea if we look at a few examples of the first law of thermodynamics.
First Law Of Thermodynamics Examples:
- Plants convert the radiant energy of sunlight to chemical energy through photosynthesis. We eat plants and convert the chemical energy into kinetic energy while we swim, walk, breathe, and scroll through this page.
- Switching on light may seem to produce energy, but it is electrical energy that is converted.
Read More: First Law of Thermodynamics
Second Law of Thermodynamics
Second law of thermodynamics states that the entropy in an isolated system always increases. Any isolated system spontaneously evolves towards thermal equilibrium—the state of maximum entropy of the system.
The entropy of the universe only increases and never decreases. Many individuals take this statement lightly and for granted, but it has an extensive impact and consequence.
Visualizing the second law of thermodynamics
If a room is not tidied or cleaned, it invariably becomes more messy and disorderly with time. When the room is cleaned, its entropy decreases, but the effort to clean it has resulted in increased entropy outside the room exceeding the entropy lost.
Read More: Second Law of Thermodynamics
The video below dives deep into the second law of thermodynamics and will help one take a closer look at how entropy explains disorderliness.

Third Law of Thermodynamics
Third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
The entropy of a pure crystalline substance (perfect order) at absolute zero temperature is zero. This statement holds true if the perfect crystal has only one state with minimum energy.
Third Law Of Thermodynamics Examples:
Let us consider steam as an example to understand the third law of thermodynamics step by step:
- The molecules within it move freely and have high entropy.
- If one decreases the temperature below 100 °C, the steam gets converted to water, where the movement of molecules is restricted, decreasing the entropy of water.
- When water is further cooled below 0 °C, it gets converted to solid ice. In this state, the movement of molecules is further restricted and the entropy of the system reduces more.
- As the temperature of the ice further reduces, the movement of the molecules in them is restricted further and the entropy of the substance goes on decreasing.
- When the ice is cooled to absolute zero, ideally, the entropy should be zero. But in reality, it is impossible to cool any substance to zero.
Read More: Third Law of Thermodynamics
Thermodynamics Examples in Daily Life
Whether we are sitting in an air-conditioned room or travelling in any vehicle, the application of thermodynamics is everywhere. We have listed a few of these applications below:
- Different types of vehicles such as planes, trucks and ships work on the basis of the 2nd law of thermodynamics.
- The three modes of heat transfer work on the basis of thermodynamics. The heat transfer concepts are widely used in radiators, heaters and coolers.
- Thermodynamics is involved in the study of different types of power plants such as nuclear power plants, thermal power plants.
Thermodynamics – Summary and Overview
→ In simple terms, thermodynamics deals with the transfer of energy from one form to another . → The laws of thermodynamics are:
- First law of thermodynamics: Energy can neither be created nor be destroyed, it can only be transferred from one form to another.
- Second law of thermodynamics: The entropy of any isolated system always increases.
- Third law of thermodynamics: The entropy of a system approaches a constant value as the temperature approaches absolute zero.
- Zeroth law of thermodynamics: If two thermodynamic systems are in thermal equilibrium with a third system separately, then they are in thermal equilibrium with each other.
→ Entropy is the measure of the number of possible arrangements the atoms in a system can have. → Enthalpy is the measurement of energy in a thermodynamic system.
Frequently Asked Questions – FAQs
What is the importance of the laws of thermodynamics, what is an example of negative work, can energy be destroyed or lost, fans convert electrical energy into mechanical energy – this is explained by which law, does the human body obey the laws of thermodynamics.
Stay tuned to BYJU’S and Fall in Love with Learning !

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
| PHYSICS Related Links | |
Very good explanation
Nicely explained with good videos and examples.
The videos on thermodynamics is very helpful. Thankyou
good explaination sir
Good explanation
Beneficial notes☺
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Stan's Academy
Science Rules
Worksheets Thermodynamics
Assignments and worksheets – thermodynamics.
Worksheets on thermodynamics practice problems. Download complete and submit the Worksheets and Assignments on the due dates.
Worksheets and Assignments on thermodynamics
- Solutions to Thermodynamics Worksheet
- Introductory TD stoichiometry problems
- Latent heat of Vapourization
- Calorimetry Worksheet q = mcΔt Class work
- Hesses_Law_Worksheet_Practice_with_Answers
- Data Latent Heat of Fusion and Vapourization
- Data Sheet Heats of Formation
- Data Sheet Molar Enthalpies of Formation
- Spontaneity Entropy Gibbs Free Energy
- Phase Change Assignment
- Nuclear Chemistry – Balancing Rate Binding energy
- Solution to Thermodynamics Worksheet
More Chemistry Topics Links
- Chemistry Page

Share this:
- Electrochemistry
- Thermodynamics
- Rates of Rxn
- Chemical Equilibrium
- Acids & Bases
- Organic Chemistry
- ORGANIC PREP
- Science Quizzes
- Video Lessons And Quizzes
- BoylesLawLab
- Order of a reaction
- KMnO4 Titration
- Equilibrium Pblm 01
- Kc using ICE table
- Generating ICE tables
- Hetro & Homogeneous
- Law of Mass Action
- Kc Quadratic Pblm
- Activation Energy – Ea
- Effect of Conc
- Effect of Tempt
- Initial Rate Pblm
- Mechanism in RXN
- Faradays Laws
- IUPAC Naming
- Assignments to submit
- Thermodynamics Engineering: University Assignments

Unraveling Thermodynamics Engineering University Assignment Topics

Thermodynamics engineering is a foundational pillar of mechanical engineering, delving into the intricate world of energy transfer, conversion, and utilization. The application of thermodynamics principles spans across a wide range of industries, from power generation to refrigeration, and serves as a cornerstone for many mechanical engineering endeavors. As students venture into the realm of thermodynamics engineering, they encounter a diverse array of topics that lay the groundwork for their comprehensive understanding. This blog aims to help you to write your thermodynamics engineering assignment and delve into these multifaceted subjects while shedding light on the various assignments that are typically assigned within university setting s .
The Laws of Thermodynamics
At the core of thermodynamics lies a set of fundamental laws that serve as the bedrock for understanding the behavior of energy in various forms. Within the realm of thermodynamics engineering, students are tasked with delving deeply into these laws and uncovering their real-world applications. Assignments in this captivating area require a meticulous exploration of these laws, prompting students to decipher their significance within practical contexts. As part of these assignments, students often grapple with intricate concepts, such as:

- Carnot Cycle Efficiency Analysis: Through this assignment, students embark on a journey to calculate and meticulously evaluate the efficiency of the Carnot cycle. This theoretical construct stands as a beacon, representing the uppermost threshold of efficiency attainable for heat engines. As students engage in this analysis, they gain insights into the intricate balance between temperature differentials and energy conversion, laying the groundwork for their understanding of practical heat engine systems.
- Second Law Limitations Discussion: Venturing further into the world of thermodynamics, students delve into the constraints and implications imposed by the second law of thermodynamics. This assignment prompts students to shed light on profound concepts, including the inexorable rise of entropy and the notion of energy degradation. As they dissect these limitations, students grasp the essential role that entropy plays in dictating the direction and feasibility of energy transformations, a knowledge crucial for designing efficient systems.
These assignments provide students with an immersive experience, allowing them to unearth the intricacies of these laws and their profound impact on the engineering landscape. By engaging in Carnot cycle efficiency analysis and unraveling the intricacies of the second law, students cultivate a deeper appreciation for the underlying principles that govern energy interactions, paving the way for their journey into the realm of thermodynamics engineering.
Thermodynamic Systems and Processes
In the intricate tapestry of mechanical engineering, thermodynamic systems and processes emerge as foundational threads that engineers deftly weave to design and optimize a plethora of mechanical marvels. As students embark on their educational journey within the realm of thermodynamics engineering, they find themselves immersed in the study of these fundamental concepts. Assignments within this domain serve as a gateway to understanding the nuanced intricacies of thermodynamic systems and processes, encompassing tasks such as:
- Process Analysis: The assignment beckons students to embark on a captivating journey of analysis, where they unravel the mysteries of various thermodynamic processes. From the controlled equilibrium of isothermal processes to the energy-conserving nature of adiabatic transformations and the idealized isentropic transformations, students delve into the nuances of each process type. Through meticulous examination, they grasp the pivotal role that process selection plays in shaping the behavior of thermodynamic systems. These analyses serve as the crucible through which students forge a deep-seated understanding of how energy and matter flow within mechanical systems.
- Properties Change Calculation: Within this assignment, students don the mantle of explorers as they navigate the uncharted territories of property changes within thermodynamic processes. With analytical precision, students meticulously calculate and compare alterations in critical properties—internal energy, enthalpy, and entropy—across diverse thermodynamic processes. By deciphering these transformations, students gain a profound insight into the dynamic interplay between energy states and the inherent behavior of matter. This foundational knowledge equips them with the tools to predict and optimize the performance of thermodynamic systems, enabling them to design with precision and finesse.
These assignments serve as vessels of discovery, propelling students into the heart of thermodynamic systems and processes. By engaging in process analysis and unraveling the calculus of property changes, students forge a strong foundation upon which they can construct their future contributions to mechanical engineering. With each assignment, they inch closer to unraveling the intricate fabric of thermodynamics, paving the way for their evolution into adept engineers capable of sculpting innovative and efficient mechanical systems.
Steam Power Cycles
In the realm of energy generation, steam power cycles emerge as the stalwart workhorses that fuel the engines of modern civilization. At the epicenter of this intricate domain, students of thermodynamics engineering find themselves on a quest to unravel the mysteries of steam power cycles. These captivating assignments serve as the crucible through which students forge their understanding of steam-driven systems, paving the way for innovations that will illuminate the future. Within this captivating landscape, students might navigate assignments that include:
- Rankine Cycle Efficiency Enhancement: Embarking on a journey of innovation, students delve into the heart of the Rankine cycle—an essential cornerstone of power generation. This assignment beckons students to channel their creativity and engineering acumen to explore techniques that enhance cycle efficiency. Through the strategic application of concepts such as superheating and regeneration, students unlock the potential to push the boundaries of efficiency, elevating the performance of steam power systems. In this pursuit, they align themselves with the pioneers of energy technology, devising solutions that shape the course of electricity generation.
- Irreversibility Impact Analysis: As students tread the path of exploration, they encounter a concept of paramount importance: irreversibility's. With the meticulous eye of a detective, students scrutinize the effects of these irreversibilities—manifested as friction, heat losses, and other dissipative processes—on the performance of steam power cycles. Armed with the tools of thermodynamics, students unravel the intricate tapestry of cause and effect, quantifying the toll that irreversibilities exact on efficiency and energy conversion. This analysis equips them to engineer systems that mitigate losses, optimizing the balance between energy input and useful output.
In the crucible of these assignments, students harness the power of inquiry and analysis, propelling themselves into the vanguard of energy innovation. Through their explorations in Rankine cycle efficiency enhancement and their dissection of irreversibility's impact, students emerge as architects of change, poised to redefine the landscape of electricity generation. As they manipulate the levers of efficiency and dissect the nuances of performance, students contribute to the evolution of steam power cycles—a testament to the enduring relevance of thermodynamics engineering in shaping the world's energy future.
Refrigeration and Heat Pump Cycles
In the intricate symphony of temperature control, refrigeration and heat pump cycles take center stage, conducting a harmonious dance that orchestrates the very essence of modern living. As students of thermodynamics engineering embark on their quest to unravel the mysteries of controlled thermal environments, they find themselves immersed in assignments that illuminate the artistry of refrigeration and heat pump systems. Within this realm of temperature mastery, students encounter assignments that span the spectrum of innovation and understanding:
- Vapor-Compression Analysis: In this assignment, students assume the roles of virtuoso conductors, tasked with evaluating the intricate performance of vapor-compression refrigeration cycles. With keen analytical prowess, they scrutinize every note of this thermal symphony, calculating metrics that unveil the cycle's efficiency and effectiveness. A highlight of this analysis is the computation of the coefficient of performance (COP), a measure that quantifies the cycle's ability to extract heat from low-temperature environments. Through this evaluation, students emerge with a profound appreciation for the delicate balance between energy input and cooling output, enabling them to fine-tune systems that serve as the lifeblood of refrigeration technology.
- Reversible vs. Irreversible Processes: As students delve deeper into the realm of refrigeration and heat pump cycles, they encounter a pivotal concept: the duality of reversible and irreversible processes. Through thoughtful discourse and analysis, students engage in a scholarly exploration of the nuances inherent to these processes within the context of refrigeration systems. They grasp the profound implications of reversibility, acknowledging its role in idealized thermodynamics, and juxtapose it against the real-world complexities of irreversible processes. This nuanced discussion equips students to navigate the dynamic landscape of refrigeration, aligning their understanding with the practical constraints that shape the design and operation of these essential systems.
In the crucible of these assignments, students emerge as adept practitioners of thermal manipulation, capable of wielding the principles of thermodynamics to sculpt controlled environments. Through their mastery of vapor-compression analysis and their exploration of reversible and irreversible processes, students harness the power to shape the very fabric of modern comfort and convenience. As they traverse the realms of temperature regulation, students contribute to the legacy of innovation that fuels the advancement of refrigeration and heat pump cycles—a testament to the enduring relevance of thermodynamics engineering in the tapestry of human progress.
Gas Mixtures and Psychometrics
In the world of HVAC (Heating, Ventilation, and Air Conditioning), the delicate interplay of gas mixtures and psychrometrics reigns supreme, acting as the masterful artisans that shape the very atmosphere of human comfort. For students treading the path of thermodynamics engineering, these concepts become the cornerstones of assignments that navigate the intricacies of air manipulation. Within this realm of ambient control, students embark on assignments that embody both scientific analysis and creative design:
- Gas Mixture Property Calculation: In a dance of molecules, students immerse themselves in the calculations that define the properties of gas mixtures—chiefly, the specific humidity and relative humidity. These assignments require a deft command over thermodynamic principles as students quantify the moisture content of air and the ratio of water vapor to the maximum amount the air could hold at a given temperature. This meticulous analysis holds profound implications for air conditioning systems, as the accurate determination of these properties dictates the system's capacity to regulate humidity and optimize comfort levels for occupants.
- HVAC System Design: Transforming from analysts to designers, students transition into the realm of HVAC system creation. In this assignment, they wield their understanding of psychrometric properties as they engineer efficient air conditioning systems for specific spaces. By seamlessly integrating concepts of temperature, humidity, and air flow, students craft systems that balance energy consumption with thermal comfort. This exercise challenges them to harmonize the practicalities of engineering with the nuances of human comfort, translating theoretical knowledge into tangible solutions that enhance living and working environments.
Through these assignments, students become maestros of atmospheric orchestration, blending science and design to create environments that nurture well-being. By mastering gas mixture property calculations and delving into HVAC system design, students contribute to the artistry of thermodynamics engineering—shaping spaces where individuals thrive and find respite. As they cultivate a deep understanding of gas mixtures and psychrometrics, students emerge poised to revolutionize HVAC technologies, shaping a world where comfort and efficiency coalesce seamlessly.
Combustion and Thermodynamic Analysis of Engines
In the heart of internal combustion engines, a fiery symphony of combustion orchestrates the dance of power and propulsion. Aspiring thermodynamics engineers step onto the stage of energy transformation, where assignments unravel the intricate dynamics of combustion and its impact on engine performance. Within this realm of controlled chaos, students find themselves immersed in assignments that merge science and engineering to ignite innovation:
- Combustion Thermodynamics: Armed with thermodynamic principles, students embark on a voyage through the fiery world of combustion. In this assignment, they dissect the intricacies of combustion reactions, analyzing the energy transfers, heat release, and temperature profiles that govern this elemental process. Through calculations of air-fuel ratios, students unveil the delicate balance required for efficient combustion—a fusion of oxygen and fuel that propels engines forward. As they predict engine performance parameters such as power output and thermal efficiency, students bridge the gap between theory and real-world engine dynamics, gaining insights that fuel the design of powerful and efficient combustion systems.
- Compression Ratio Optimization: Shifting gears from the combustion process itself, students delve into the art of engine optimization through compression ratios. With an engineer's precision, they explore the profound impact of compression ratios on engine efficiency and performance. Through analysis and simulation, students uncover the delicate equilibrium between compression ratios and thermal efficiency, unraveling the trade-offs that dictate power output, fuel consumption, and emissions. Armed with this knowledge, students embark on a journey of discovery, seeking the sweet spot that maximizes engine performance while minimizing environmental impact.
In the crucible of these assignments, students harness the power of combustion as a force of transformation and propulsion. Through the analysis of combustion thermodynamics and the exploration of compression ratio optimization, they emerge as architects of energy conversion, sculpting engines that merge power, efficiency, and sustainability. As they dive into the inferno of combustion and its thermodynamic intricacies, students stand at the precipice of engine innovation, poised to ignite a future where internal combustion engines become beacons of power and environmental stewardship.
In the realm of thermodynamics engineering, a captivating blend of theoretical concepts and practical applications awaits students. The diverse range of topics covered equips them with the knowledge to tackle complex engineering challenges and contribute to energy-efficient solutions. Through a plethora of assignments spanning from analyzing fundamental laws to exploring renewable energy systems, students emerge with a profound understanding of thermodynamics engineering, poised to shape a future driven by sustainable engineering practices.
Post a comment...
Exploring thermodynamics engineering: unraveling university assignments and topics submit your assignment, attached files.
| File | Actions |
|---|
Homework Q&A
Drag image here or click to upload
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History

Chemistry AI Solver
HIX Tutor's AI chemistry homework helper is your go-to companion to master chemistry problem-solving.

Get Instant Chemistry Homework Help At HIX Tutor
HIX Tutor's AI-powered chemistry homework solver is the ultimate companion for completing your chemistry assignment. Effortlessly solving complex reaction calculations and offering detailed problem solutions, this is an indispensable tool for students seeking clear, instant, and accurate assistance in chemistry studies.
Cutting-Edge Features of Our Chemistry AI
All-round chemistry support.
From basic concepts to complex chemistry problems, our AI chemistry equation solver provides thorough assistance for all levels.
Step-by-Step Solutions
The AI chemistry homework solver breaks down complex equations into understandable steps, offering detailed guidance to enhance learning.
Efficient and Economical
Streamline your study sessions with our efficient AI chemistry problem solver, saving valuable time and resources while achieving better academic results.
Multilingual Support
HIX Tutor’s AI chemistry homework helper is available in multiple languages, ensuring no student is left behind due to language barriers.
Available 24/7
Day or night, our AI chemistry problem solver is always ready to assist, providing reliable and instant chemistry help whenever you need it.
Worldwide Accessibility
Our chemistry AI problem solver is universally accessible, offering consistent and effective chemistry solutions to students worldwide.
How to Get Quick Chemistry Homework Help From HIX Tutor?
Experience the transformative power of HIX Tutor's AI chemistry homework solver with the following easy steps:
Initiate Your Chemistry Inquiry
Enter or upload your chemistry assignment questions into HIX Tutor's Chemistry AI homework solver to get started.
Experience AI-Driven Problem Solving
Once you input your query, our chemistry equation solver will use AI algorithms to dissect and process your question.
Obtain Comprehensive Solutions
Our chemistry helper will generate step-by-step explanations, making complex concepts and reactions understandable.
Other AI Homework Helpers
From basic arithmetic to advanced calculus, get understandable steps for complex math problems.
Demystify topics ranging from basic mechanics to advanced electromagnetism with detailed solutions
Clarify doubts, and get insights on complex processes and terminologies. Get accurate answers to hard.
The Dynamic Capabilities of Our Chemistry AI
| 🧪 Chemistry expertise | Accurate solutions instantly |
|---|---|
| ⏰ Always ready to assist | Reliable problem-solving 24/7 |
| 🌍 Global educational access | Worldwide reach and use |
| 🔍 Detailed solution breakdown | Easy-to-understand explanations |
| 💡 Time-saving study aid | Quick, efficient homework help |
1. What is a Chemistry AI?
A Chemistry AI applies artificial intelligence techniques to solve complex chemistry problems, such as predicting reaction outcomes, designing novel compounds, or optimizing synthesis pathways. By using AI algorithms, Chemistry AI can streamline research and development processes in drug discovery, materials science, and other areas of chemical research.
2. Can this chemistry problem solver tackle diverse homework queries?
Yes, it's equipped to handle a range of chemistry homework questions, from basic concepts to advanced topics like stoichiometry and thermodynamics. While highly specific or research-level queries might have limitations, it effectively covers most educational levels.
3. How accurate is the HIX Tutor chemistry homework helper?
Empowered by state-of-the-art AI, HIX Tutor's chemistry homework helper boasts a wide-ranging chemical knowledge base, and it is able to provide accurate and reliable solutions across a vast array of chemistry topics.
4. Is HIX Tutor’s chemistry problem solver suitable for various academic levels?
Absolutely. HIX Tutor's chemistry AI homework solver is versatile enough to aid students across different academic levels. From high school chemistry to college-level assignments, it provides valuable support, enhancing understanding for students of all academic levels.
5. Is this chemistry AI free?
Yes, our Chemistry AI Solver is indeed available for free. You can solve chemistry problems without incurring any costs.
6. What's the best Chemistry AI?
HIX Tutor is the best chemistry solver designed to assist students and researchers in solving complex chemistry problems. It offers a wide range of features, including step-by-step solutions and detailed explanations of key concepts. With HIX Tutor, users can better understand chemical reactions, improve problem-solving skills, and gain a deeper understanding of the subject matter.
Discover Frequently Asked Chemistry Questions and Their Answers
- Formula for Cd2+ and PO43- ? also whats its name
- What is the Lewis structure for a perchlorate ion?
- Which of these structures has dipole-dipole interactions? Water (H2O), ethyl alcohol (CH3CH2OH), and hexane (C6H14).
- Is a single molecule of oxygen held together by two nonpolar covalent bonds?
- Why do the alkali metals have to be stored under oil?
- How do you know the number of electrons in the outermost energy level of an atom?
- How do you define a valence electron? How do you determine the valence number of an atom?
- Do ionic bonds occur between atoms from adjacent groups?
- Why are the melting point and boiling point of graphite so remarkably high?
- Which statement is INCONSISTENT with Dalton's atomic theory?
- What is Ka for #HClO_4#?
- How do you rank Bronsted acids?
- Which is the stronger acid in each of the following pair #H_2SeO_3# or #H_2SeO_4#?
- Which is the stronger acid in each of the following pair #HBrO_2# or #HBrO#?
- How do you draw the cis and trans isomers for 1-ethyl-3-methylcyclobutane?
- How does a hydrolysis reaction separate a polymer into its monomers?
- Why are there cis and trans isomers?
- What does it mean for a molecule to be optically active?
- What is the most stable conformer for 3,3-dimethylhexane, viewed along the #C_3-C_4# bond using Newman projections?
- Which group contains a chiral carbon? #A.# #"1-bromopropane"#. #B.# #"2-bromopropane"#. #C.# #H_3C−CH_2CHBrCH_2CH_3#. #D.# #"cyclohexyl bromide"#

Ease Your Chemistry Learning Journey With Our Chemistry AI Problem Solver
HIX Tutor’s AI chemistry homework helper is your reliable partner for assignment help and exam preparation. Try it now and experience a new standard in AI-powered education.
- Implementation Reference
Example of Running Lead Batch Assignment in Diagnostic Mode
You can run your batch assignment in diagnostic mode to view the details of the assignment processing in an output log. This topic provides an example of running lead batch assignment in diagnostic mode.
Running Batch Assignment
A sales representative of a company has to follow up on a lead but the lead hasn't yet been assigned to his territory. He has requested you, the sales administrator, to investigate the details of territory assignment. You can provide these details by running lead batch assignment in diagnostic mode.
Go to Navigator , and then select the Lead Qualification menu item.
Select Lead Processing Activities on the Tasks pane.
On the Lead Processing Activity page, click the Create Lead Processing Activity button.
On the Create Lead Processing Activity page:
Select Assignment from the Process Type list.
Enable diagnostic mode by checking the Diagnostic Mode check box.
Search and select a lead. Note down the lead number value to use in a later step.
Select Immediate from the Schedule list.
Click Submit .
On the Confirmation dialog box, click OK .
Two process are submitted, one for lead territory assignment and the other for lead rule-based (or resource) assignment. Note down the identifier of the territory or resource assignment processes you're interested in.
Click the Refresh icon until the process has completed successfully or with an error.
Select the appropriate territory or rule-based assignment process, and then click the Output log icon in the View Log column to view details.
Open the log file in another browser window or tab.
View the log file for details of the assignment processing for the selected lead. You can use the lead number recorded earlier to search in the log file. Review the file for details of the assignment processing.
Related Topics
- Batch Assignment Diagnostic Log

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Open Search
Mobile Menu
BLM In Belize: Employees Participate in International Assignment Taking Them From Districts of Utah to Caves of Central America
Located just beyond the shared Belizean and Guatemalan border, the BLM team consisting of Savanna Agardy and Kyle Voyles found themselves navigating through thick jungle and looking ahead to the prospect of six days of work mapping one of the largest cave systems in the world with the help of Friends for Conservation and Development (FCD), Department of Interior (DOI) and the International Technical Assistance Programs (ITAP) cave experts.

Spider Monkeys swinging overhead, beetles flying around at dusk, and the alarm clock of parrots singing at sunrise. It sounds nothing like the experience of a typical Utah resident but, instead, a tale from BLM cave explorers that begins with baths in the caves, fresh-flowing water, and nights ending in swaying hammocks.
After waking up to the distinct sounds of parrots and toucans and with the occasional late-night Howler Monkey call, the team got to work in what Agardy describes as “an experience of a lifetime”.
Packing up camp and moving into the unknown Agardy, Voyles and accompanying members from the Belizean Institute of Archaeology (the government agency that oversees all of Belize's archaeological heritage) successfully mapped a remote section of the Chiquibul Cave System (CCS) measuring an approximate mile. While this task seems daunting by itself, mapping wasn't the only assignment at hand: the team additionally identified 50 archaeological features and over a dozen artifacts from 200-900 AD, a time frame known as the Classic Maya Period.

After learning all they could from the story that the CCS had to tell, it was time that the team turned their focus towards teaching, walking the FCD staff through cave rescue methods with help from Gretchen Baker (DOI-ITAP team member) and Kyle Rybacki, a former BLM employee.
After a long flight bringing the team back to Utah, Agardy and Voyles continue to work on their findings, with some help from digital technology in designing maps and reporting archeological finds.
All this effort would be futile without help from the DOI-ITAP with the support of the U.S. Agency for International Development/Guatemala , which assists and encourages our teams' continued exploration efforts.
With skies covered by jungle canopies and caves of mysterious heritage still left to explore, visit https://www.doi.gov/itap/opportunities to find out how to get in on the action and experience the unmapped regions of the earth calling to be discovered and questioned.

Thomas Cogdell, BLM Utah Public Affairs Intern
Blog Topic:
Related stories.
- July 31, 2024 BLM Archaeology in Action
- July 16, 2024 Ripple Effect: Dillon Field Office Partners Help Riparian Areas Thrive
- July 17, 2024 BLM Monticello Field Office host first Junior Ranger Day Event
- June 28, 2024 BLM’s Fort Stanton Cave focus of talk at Smokey Bear Days
- June 25, 2024 BLM Utah West Desert District Donates Water Tender to North Tooele Fire District
Browse Course Material
Course info.
- Prof. Rafael Jaramillo
Departments
- Materials Science and Engineering
As Taught In
- Thermodynamics
Learning Resource Types
Thermodynamics of materials, communications (ci-m) assignments.
Please refer to the calendar section to see where the assignment due dates fall in the class schedule. These assignments are part of the CI-M aspect of the course.
Literature Review Assignment Guidelines
Description.
Over the course of this assignment, you will write and revise a short literature review.
As a stand-alone genre, literature review articles are published to analyze the state of a specialized field, by synthesizing recent research and identifying patterns, trends, and remaining open questions. You can think of them as a public service researchers perform for their community, as it is then easier for the rest of us to digest recent research quickly, and to identify the particular articles that would be most relevant for our own work. Literature reviews are not entirely selfless acts, however, because they are also an opportunity for researchers to shape future research agendas by evaluating and critiquing the current trends. For your review, you’ll focus on objectively summarizing research trends, but you may include any evaluative reflections, if relevant, in your conclusion.
Shorter, more targeted literature reviews are also generally required as a component of most research articles’ Background or Related Works sections.
Your task is to write a 2,000-word literature review that synthesizes recent research on an aspect of the field of thermodynamics (a more focused subtopic within the one you presented on).
When we evaluate your final draft, we will be assessing how effectively you do the following:
- provide your fellow researchers an overview of the current trends, promising approaches, and remaining open questions in the field
- understand and address your audience (considering their knowledge and expectations in your framing of content, structure, and discourse)
- synthesize and integrate evidence from sources
Research Process
To be successful, authors of literature reviews need to keep careful track of the novel contributions, claims, and open questions associated with their different sources, as well as observe and track trends, patterns, and critical responses.
For this assignment, you will need to focus your research, select 7–12 peer-reviewed articles to include in an annotated bibliography, and ultimately engage in close synthesis of 5–10 of these articles in your final draft.
We recommend using tools like a literature review chart , Mendelay or Zotero, as well as your course librarian as you navigate the research.
Drafting the Assignment
Ultimately, your literature review should present the following sections coherently:
1. Introduction
Motivates the reader’s interest by establishing the significance and challenges of your topic, identifies the purpose of providing a summary overview of recent research, and provides a conceptual framework that organizes the material logically, highlighting important concerns and how they relate to each other.
2. Body Paragraphs
Arranged in a logical sequence, body paragraphs should identify the major points of intersection between sources and be structured to closely synthesize information and clearly link specific examples and evidence to the various concepts at play, aiding the reader in understanding their significance. More broadly, body paragraphs must define central concepts clearly (noting how different articles use them, if relevant), provide an accurate representation of the different articles, and give a comprehensive summary of the main issues.
3. Conclusion
This briefly reframes your review’s main points, focusing on key intersections between sources and open questions or directions for the field.
4. Figures + References
Include at least 1–2 figures and/or tables (titled, labeled, captioned, and referenced in the text) to illustrate complex concepts. Cite all source material with an in-text reference and in a full reference page (use Nature’s style guide).
Presentation Assignment Guidelines
Over the course of this assignment, you will prepare a 10-minute presentation on a thermodynamics-related topic of your choice (e.g. phase diagrams for thermal storage materials, the role and importance of computational materials design) for a general audience. The context and motivation for your presentation is a (hypothetical) Museum of Science speaker series showcasing student research topics at MIT. The series is aimed at strengthening public support and understanding of science.
To support your 10-minute talk, you will need to design a short slide deck of approximately 8–15 slides. These slides should contain visuals and text that complement and clarify the key concepts in your talk (as well as provide references) and should be designed to accommodate your target audience.
During conferences in weeks 4 and 5 (to be scheduled in week 3), you will have the opportunity to workshop and practice your presentations in small groups (please bring a rough draft of your slides and talk, or whatever materials you have, to your conference for constructive feedback).
You will give your 10-minute final presentation to a group of peers and instructors, followed by questions and discussion.
When we evaluate your presentation, we will be assessing how effectively you do the following:
- communicate complex ideas clearly in visual and oral form
- design figures to convey and emphasize key concepts
SELECTING A TOPIC
As you consider selecting a course-related presentation topic, keep in mind that the topic you select for this assignment will also carry over to the next CI-M assignment, the literature review. Feel free to select one of the topics listed below, or propose your own topic by sending a short email to your instructors.
Presentation Topics:
- Usefulness of eutectic systems
- Thermodynamics of phase transformations
- Role and importance of computational materials design
- Phase diagrams for materials selection and processing
- Sustainable materials selection
Possible Literature Review Topics:
- Deep eutectic solvents in drug delivery systems
- Phase change energy storage
- Computational microstructure characterization and reconstruction
- Extraction and processing of rare earth metals
- Materials for solar energy capture and conversion
In order to approach and present your chosen topic, you will need to perform independent research. Ideally, the research process for your presentation will become useful background as you focus your research for the literature review.
PREPARING YOUR PRESENTATION
In a rhetorically strategic and coherent structure, your presentation should include the following elements:
- Motivation: Why should the audience care about this research? What are the real-world applications and stakes? What gaps or frontiers in research are scientists trying to fill or extend?
- Context: What does the audience need to know to understand key terms and concepts?
- Methods: How do methods help scientists accomplish goals? How did the field develop these methods?
- Results and Open Questions: What is the current state of the art? What are the open questions in the field?
- Conclusion: What main ideas from the talk do you want to highlight and leave your audience remembering?
- Figures + References: How can visuals and graphics clarify, enhance, or support each of these goals? Remember to cite all references.

You are leaving MIT OpenCourseWare
The new 'Swift Boat?' Tim Walz's military service targeted by Trump campaign
WASHINGTON – Democratic vice presidential nominee Tim Walz's Army National Guard service has become an immediate target of Republican Donald Trump's campaign, with running-mate JD Vance, also a veteran, attacking Walz's retirement from the military prior to his battalion's deployment to Iraq.
Walz, the governor of Minnesota and a former congressman, joined the National Guard out of high school at 17 years old on April 8, 1981 and served until May 16, 2005. Walz has said he retired from the guard to run for Congress, which he did successfully in 2006.
The alert order for Walz's unit to mobilize for Iraq was received on July 14, 2005 − almost two months after Walz had retired − and the unit mobilized Oct. 12, 2005, according to the Minnesota National Guard. But according to past press statements, Walz appeared to know as early as March 2005 that his battalion could be sent to Iraq.
More: Why Kamala Harris chose Tim Walz over Josh Shapiro as her running mate
Vance raised Walz's departure from the military Wednesday while campaigning in Shelby Township, Michigan, comparing their military records. Vance joined the Marines in 2003 and served as a combat correspondent – or military journalist – until 2007. Vance was deployed to Iraq for six months in late 2005 to serve in public affairs.
"When the United States of America asked me to go to Iraq to serve my country, I did. I did what they asked me to do, and I did it honorably, and I'm very proud of that service," Vance said. "When Tim Walz was asked by his country to go to Iraq, you know what he did? He dropped out of the army and allowed his unit to go without him."
Vance added: "What bothers me about Tim Walz is the 'stolen valor' garbage. Do not to pretend to be something you're not."
Vice President Kamala named Walz her running-mate Tuesday , with the campaign highlighting his Midwest, working-class biography and military service.
Vance's attacks have drawn comparisons to the unsubstantiated "Swift Boat" claims against Democrat John Kerry in the 2004 presidential race against President George W. Bush. The group Swift Boat Veterans for Truth challenged Kerry's decorated Vietnam War record. Chris LaCivita, a senior adviser to the Trump campaign, led the Swift Boat campaign attacking Kerry in 2004 .
Vance seized on past remarks from Walz in support of gun control shared Tuesday on social media by the Harris campaign in which the governor said, "We can make sure that those weapons of war that I carried in war is the only place where those weapons are at."
"I wonder, Tim Walz, when were you ever in war?" Vance said Wednesday. "What was this weapon that you carried into war given that you abandoned your unit right before they went to Iraq?"
In a statement to USA TODAY, Harris campaign spokesman Ammar Moussa reiterated that Walz retired in 2005 to run for Congress, where he later chaired the House Veterans Affairs Committee.
More: Tim Walz's military record: What to know about potential VP's National Guard service
More: Tim Walz debuts as Kamala Harris' VP pick at raucous Philly rally: Recap
In response to Vance saying Walz never carried a weapon into war, Moussa said: "In his 24 years of service, the Governor carried, fired and trained others to use weapons of war innumerable times. Governor Walz would never insult or undermine any American's service to this country − in fact, he thanks Senator Vance for putting his life on the line for our country. It's the American way."
A review of Walz's old website from his run for Congress − still preserved online − shows a March 20, 2005, news release from the Walz congressional campaign stating that the National Guard public affairs office on March 17 of that year announced a "possible partial mobilization of roughly 2,000 troops from the Minnesota National Guard."
According to the archived news release, the public affairs office notified that "all or a portion of Walz's battalion could be mobilized to serve in Iraq within the next two years."
Walz is quoted in the release saying in March 2005, "I don't want to speculate on what shape my campaign will take if I am deployed, but I have no plans to drop out of the race." The final line in the news release reads, "If called to duty, Walz would leave behind his wife Gwen and four year old daughter, Hope."
Moussa said the Harris campaign had no further comment.
Former Republican Rep. Adam Kinzinger of Illinois − a Trump critic, Harris supporter and veteran of the Air National Guard − pushed back at Vance's criticism.
"JD served honorably, but he wasn’t kicking down doors. He was in public affairs. Which again, is fine and honorable. Tim, after he was eligible for retirement, retired. People do that," Kinzinger wrote on X, formerly Twitter. "If it was a real problem he would have been 'stop lossed' and prevented from retiring."
A stop-loss refers to the involuntary extension of an individual's activity duty service enforced by the Defense Department.
"If JD Vance is going to go after Tim Walz, I would suggest he take from his own military training, his own military experience," said Maryland Gov. Wes Moore, a Harris surrogate and also a military veteran. "Do not start with trying to attack that someone raised their hand to serve this country."
Walz rose to the rank of command sergeant major, but because he did not complete additional course work at the U.S. Army Sergeants Major Academy before leaving the National Guard in 2005, he retired as a master sergeant.
"You get to sergeant major because you have a track record of taking care of your people," Moore said in an interview on MSNBC .
Walz mobilized with the Minnesota National Guard on Aug. 3, 2003, in support of Operation Enduring Freedom, whose primary mission was the Afghanistan War but also had counterterrorism roles in other regions.
His 1st Battalion of the 125th Field Artillery supported security missions in Europe and Turkey, according to a statement from Lt. Col. Kristen Auge, a spokesperson for the Minnesota National Guard.
Walz was stationed at Vicenza, Italy, during his deployment and returned to Minnesota in April 2004, Auge said.Walz started his service in the Nebraska National Guard and finished at the Minnesota National Guard.
More: 'Resurrected from the dead': Harris pick of Walz caps complete shake-up of 2024 race
Walz's Republican opponents in Minnesota have criticized his exit from the military in the past, including during his 2018 and 2022 runs for governor.
In an open letter in 2018 , Minnesota National Guard veteran Thomas Behrends accused Walz of pushing "embellished and selectively omitted facts" about his military service. The Minneapolis Star-Tribune reported that Behrends was passed over for the promotion to command sergeant major that went to Walz and described him as a "longtime critic of the governor."
Retired Maj. Gen. Randy Manner, who held top positions with the National Guard Bureau and said he does not know Walz but plans to vote for the Harris-Walz ticket, called the controversy manufactured. Walz would have filed his papers for retirement long before his unit received official notice that they were being mobilized for deployment. The retirement papers Walz filed take five to nine months to process, Manner said.
“The idea that a man who served his country honorably for 24 years somehow ‘cut and run’ because he retired from the Guard in his 40s to pursue his next phase of life is an insult to him and everyone else who also served their nation so honorably," Manner said.
Walz acknowledged he never saw combat in a 2018 interview with Minnesota Public Radio .
"I know that there are certainly folks that did far more than I did. I know that," Walz said at the time. "I willingly say that I got far more out of the military than they got out of me, from the GI Bill to leadership opportunities to everything else."
Reach Joey Garrison on X, formerly Twitter, @joeygarrison.

IMAGES
COMMENTS
Assignment 1: Thermodynamics Useful relations: Equations of State of ideal and van der Waals gases
Assignments. In addition to a complete set of problem sets with solutions, weekly example problems with solutions are available below.
Thermodynamics is the study of how heat moves around in 'macroscopic' objects. Through-out these lectures, we will talk a lot about laws and models. Models are a simplified, empirical description of a real system which generally develops overtime as our knowledge progresses.
This page includes problem set, recitation problems, and assignments related to the communications requirement of the course.
This page includes 3 problem sets with solutions.
This unit examines the role of energy in physical and chemical processes. Learn about heat transfer, calorimetry, enthalpy of reaction, Hess's law, and more. Practice what you've learned and study for the AP Chemistry exam with more than 55 AP-aligned questions.
1. Introduction Thermodynamics is the study of how heat moves around in 'macroscopic' objects. Through-out these lectures, we will talk a lot about laws and models. Models are a simplified, empirical description of a real system which generally develops overtime as our knowledge progresses. In contrast, laws derive from fundamental principles of Physics and thus apply⇤ universally ...
Basic Concepts of Thermodynamics Every science has its own unique vocabulary associated with it. Precise definition of basic concepts forms a sound foundation for development of a science and prevents possible misunderstandings. Careful study of these concepts is essential for a good understanding of topics in thermodynamics.
1 Introduction Thermodynamics is the study of heat and temperature. One thing that makes thermodynamics hard (and generally unpopular) is all the damn variables. Everything is related and it's often tough to keep straight what is an independent and what is a dependent variable. We will do our best to write the dependent variables explicitly whenever possible. Another thing that makes ...
Approaching thermodynamics and climate change assignments systematically can make complex problems more manageable. Here's a step-by-step guide to help you solve such assignments effectively: 1. Analyzing Entropy Changes: For Reversible Processes: Use the second law of thermodynamics and the definition of entropy change (ΔS=∫dQrevT\Delta S ...
Course Learning Objectives: To be able to use the First Law of Thermodynamics to estimate the potential for thermo-mechanical energy conversion in aerospace power and propulsion systems.
This section includes assigments and solutions from the kinetics segment of the course.
thermodynamics, science of the relationship between heat, work, temperature, and energy. In broad terms, thermodynamics deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.
Thermodynamics engineering assignments often delve into intricate topics such as Statistical Thermodynamics of Complex Liquids, demanding a comprehensive grasp of both foundational principles and advanced theoretical constructs. These assignments can pose significant challenges due to their nuanced mathematical formulations and the need for precise application of statistical methods to analyze ...
Thermodynamics is a branch in physics that deals with the interconversion of heat and other forms of energy. The thermodynamic laws provide a quantitative description of these quantities.
Worksheets and Assignments on thermodynamics. Solutions to Thermodynamics Worksheet. Introductory TD stoichiometry problems. Latent heat of Vapourization. Calorimetry Worksheet q = mcΔt Class work. Hesses_Law_Worksheet_Practice_with_Answers. Data Latent Heat of Fusion and Vapourization. Data Sheet Heats of Formation.
Second Law Limitations Discussion: Venturing further into the world of thermodynamics, students delve into the constraints and implications imposed by the second law of thermodynamics. This assignment prompts students to shed light on profound concepts, including the inexorable rise of entropy and the notion of energy degradation.
Yes, it's equipped to handle a range of chemistry homework questions, from basic concepts to advanced topics like stoichiometry and thermodynamics. While highly specific or research-level queries might have limitations, it effectively covers most educational levels.
Problem Set 9. pdf. 570 kB. Recitation 9 Problems. pdf. 206 kB. Problem Set 10. Over 2,500 courses & materials. Freely sharing knowledge with learners and educators around the world.
You can run your batch assignment in diagnostic mode to view the details of the assignment processing in an output log. This topic provides an example of running lead batch assignment in diagnostic mode.
Azure Administrators often come across challenges while tracking multiple Azure role assignments and removals. At present Azure provides Activity Logs but they make less sense to non-techsavy stakeholders. For example it includes Role Id, Principal Id but doesn't indicate Role names and Principal names which can make the report more readable ...
Researchers solve long-standing challenge for piezoelectric materials Date: August 6, 2024 Source: North Carolina State University Summary: Heat and pressure can deteriorate the properties of ...
Assignments Unless otherwise noted, problems assigned by number refer to corresponding problems in the course text: Tester, J. W., and Modell, Michael. Thermodynamics and Its Applications. Upper Saddle River, NJ: Prentice Hall PTR, 1996. ISBN: 9780139153563. Session numbers indicate due dates for problem sets.
The BLM team found themselves navigating through thick jungle and looking ahead to the prospect of six days of work mapping one of the largest cave systems in the world with the help of international agencies.
Presentation Assignment Guidelines Description Over the course of this assignment, you will prepare a 10-minute presentation on a thermodynamics-related topic of your choice (e.g. phase diagrams for thermal storage materials, the role and importance of computational materials design) for a general audience.
Democratic vice presidential nominee Tim Walz's National Guard service has become an immediate target of Republican Donald Trump's campaign