- States of Matter
- Avogadros Law

Avogadro's Law
What is avogadro’s law.
Avogadro’s law, also known as Avogadro’s principle or Avogadro’s hypothesis, is a gas law which states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
Avogadro’s law is closely related to the ideal gas equation since it links temperature, pressure, volume, and amount of substance for a given gas.
Table of Content
Formula and graphical representation, molar volume of a gas, examples of avogadros law, what are the limitations of avogadro’s law, solved exercises on avogadro’s law, recommended videos.
- Frequently Asked Questions – FAQs

Avogadro’s law is named after the Italian scientist Amedeo Carlo Avogadro, who suggested that two dissimilar ideal gases occupying the same volume at a given (constant) temperature and pressure must contain an equal number of molecules.
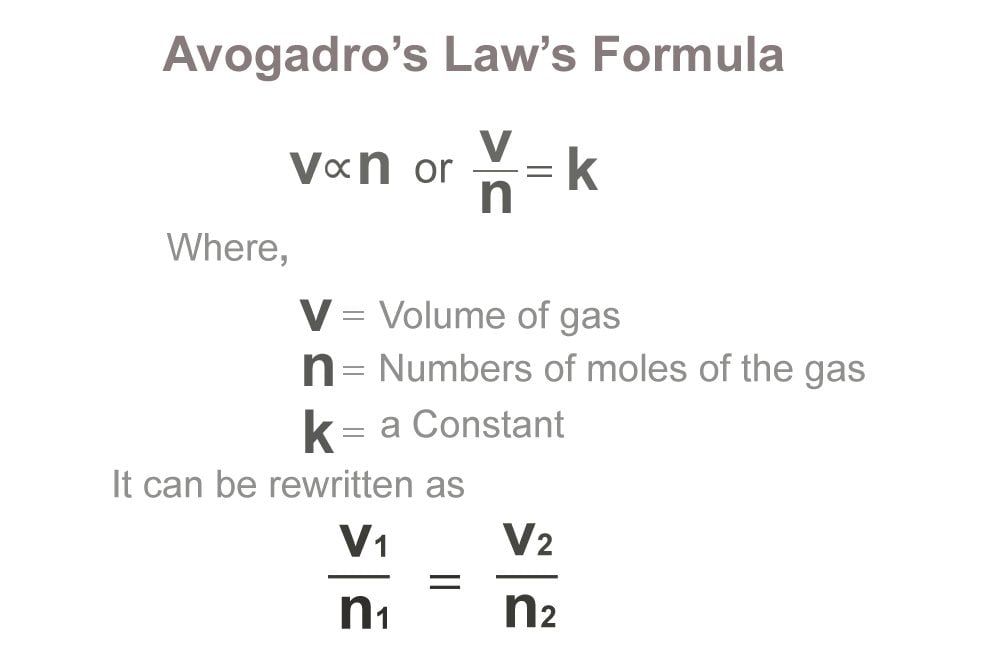
At constant pressure and temperature, Avogadro’s law can be expressed via the following formula:
Where V is the volume of the gas, n denotes the amount of gaseous substance (often expressed in moles), and k is a constant. When the amount of gaseous substance is increased, the corresponding increase in the volume occupied by the gas can be calculated with the help of the following formula:
V 1 /n 1 = V 2 /n 2 ( = k, as per Avogadro’s law).
The graphical representation of Avogadro’s law (with the amount of substance on the X-axis and volume on the Y-axis) is illustrated below.

Here, the straight line (which indicates that the two quantities are directly proportional) passes through the origin, implying that zero moles of gas will occupy zero volume.
Avogadro’s law can be derived from the ideal gas equation, which can be expressed as follows:
- ‘P’ is the pressure exerted by the gas on the walls of its container
- ‘V’ is the volume occupied by the gas
- ‘n’ is the amount of gaseous substance (number of moles of gas)
- ‘R’ is the universal gas constant
- ‘T’ is the absolute temperature of the gas
Rearranging the ideal gas equation, the following equation can be obtained.
V/n = (RT)/P
Here, the value of (RT)/P is a constant (since the temperature and pressure kept constant and the product/quotient of two or more constants is always a constant). Therefore:
Thus, the proportionality between the volume occupied by a gas and the number of gaseous molecules is verified.
As per Avogadro’s law, the ratio of volume and amount of gaseous substance is a constant (at constant pressure and temperature). The value of this constant (k) can be determined with the help of the following equation:
Under standard conditions for temperature and pressure, the value of T corresponds to 273.15 Kelvin and the value of P corresponds to 101.325 kilo Pascals. Therefore, the volume occupied by one mole of a gas at STP is:
Volume occupied by 1 mole of gas = (8.314 J.mol -1 .K -1 )*(273.15 K)/(101.325 kPa) = 22.4 litres
Therefore, one mole of any gaseous substance occupies 22.4 litres of volume at STP .
The process of respiration is a great example of Avogadro’s law. When humans inhale, the increase in the molar quantity of air in the lungs is accompanied by an increase in the volume of the lungs (expansion of the lungs). An image detailing the change in volume brought on by an increase in the number of gaseous molecules is provided below.

Another common example of Avogadro’s law is the deflation of automobile tyres. When the air trapped inside the tyre escapes, the number of moles of air present in the tyre decreases. This results in a decrease in the volume occupied by the gas, causing the tyre to lose its shape and deflate.
Despite being perfectly applicable to ideal gases, Avogadro’s law provides only approximate relationships for real gases. The deviation of real gases from ideal behaviour increases at low pressure and high temperature.
It is important to note that gases molecules having relatively low molecular masses (such as helium and hydrogen) obey Avogadro’s law to a greater extent than heavier molecules.
One mole of helium gas fills up an empty balloon to a volume of 1.5 litres. What would be the volume of the balloon if an additional 2.5 moles of helium gas is added? (Assume that the temperature and the pressure are kept constant)
The initial amount of helium (n 1 ) = 1 mol
The initial volume of the balloon (V 1 ) = 1.5 L
The final amount of helium (n 2 ) = 1 mol + 2.5 mol = 3.5 mol
As per Avogadro’s law, V 1 /n 1 = V 2 /n 2
Therefore, the final volume of the balloon (V 2 ) = (V 1 n 2 )/n 1 = (1.5L*3.5mol)/1mol = 5.25 L
The balloon would occupy a volume of 5.25 litres when it contains 3.5 moles of helium gas.
A tyre containing 10 moles of air and occupying a volume of 40L loses half its volume due to a puncture. Considering that the pressure and temperature remain constant, what would be the amount of air in the deflated tyre?
The initial amount of air (n 1 ) = 10 mol
The initial volume of the tyre (V 1 ) = 40 L
The final volume of the tyre (V 2 ) = 20 L
According to Avogadro’s law, the final amount of air in the tyre (n 2 ) = (V 2 n 1 )/V 1 = 5 moles.
The deflated tyre would contain 5 moles of air.

Frequently Asked Questions – FAQs
What does avogadro’s law state.
Avogadro’s law states that equal volumes of different gases contain an equal number of molecules under the same conditions of temperature and pressure.
Why is Avogadro’s law important?
The link between the amount of gas (n) and the volume (V) is investigated via Avogadro’s law (v). It’s a direct relationship, which means the volume of a gas is proportional to the number of moles contained in the gas sample. The law is significant because it allows us to save time and money over time.
What does Charles law state?
The physics theory known as Charles’ law asserts that the volume of a gas equals a constant value multiplied by its Kelvin temperature.
What is Avogadro’s Law in simple terms?
Why is avogadro’s law only for gases.
This is because there is so much space between each molecule that the size of the molecule has no bearing on the volume of the material. This is why the volume of a gas is governed by the pressure applied to it, and why under the same pressure, all gases have the same volume.
What are the limitations of Avogadro law?
What are the applications of avogadro law, can we apply the ideal gas law to liquids, when was avogadro’s law discovered, why was avogadro’s law rejected.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Extraordinarily great
Very helpfull. Thanks
Very helpful and great
This is very helpfull thankssssss Byjus
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Avogadro’s Law – Definition, Formula, Examples

Avogadro’s law states the volume of an ideal gas is directly proportional to the number of moles of gas , under conditions of constant temperature and pressure. As the number of moles of a gas increase, the volume increases proportionally. This is independent of the size of the gas particles or their molar mass, so gases of different elements and compounds are comparable to one another.
Of course, as with any ideal gas law , the behavior of real gases deviates slightly from predicted behavior. The law assumes each gas particle has no volume and that particles bounce off each other and their container in perfectly elastic conditions. Real gas molecules have volume and may be attracted or repelled by one another. Even so, Avogadro’s law is a useful approximation that is reasonably accurate for real gases under normal conditions.
The law is named for Amedeo Avogadro . In 1812, Avogadro hypothesized that two ideal gas samples contained the same number of molecules if they were at the same temperature and pressure. For example, a vial of hydrogen gas and a vial of nitrogen gas contain the same number of molecules at the same volume, temperature, and pressure, even though the gases have different identities.
Avogadro’s law is also known as Avogadro’s hypothesis or Avogadro’s principle. It is related to the other ideal gas laws: Boyle’s law (1662), Charles’s law (1787) and Gay-Lussac’s law (1808). French physicist and mathematician André-Marie Ampère published the same law as Avogadro, but in 1814. In France, the relation was called Ampère’s hypothesis , Avogadro–Ampère hypothesis , or Ampère–Avogadro hypothesis .
Avogadro’s Law Formula
There are four common formulas representing Avogadro’s law, where V is volume, n is number of moles of gas, and k is a constant:
V ∝ n V/n = k V 1 /n 1 = V 2 /n 2 V 1 n 2 = V 2 n 1
Because volume and number of moles are directly proportional to one another, a graph of volume versus number of moles is a straight line, extending upward from the origin.
Example of Avogadro’s Law in Everyday Life
The best example of Avogadro’s law is blowing up a balloon. The balloon’s volume increases as you add moles of gas. Similarly, when you deflate a balloon, gas leaves the balloon and its volume shrinks.
Avogadro’s Law Example Problem
A 13.5 L volume of gas contains 0.000524 moles of nitrogen gas. Assuming the temperature and pressure of the gas remain unchanged, what volume does 0.00144 moles of the gas fill?
First, write down what you know and identify the unknown value:
V 1 = 13.5 L V 2 = ? n 1 = 0.000524 mol n 2 = 0.00144 mol
Next, plug the values into the Avogadro’s law formula and rearrange the equation to calculate the answer:
V 1 /n 1 = V 2 /n 2 13.5 L / 0.000524 mol = V 2 / 0.00144 mol V 2 / 0.00144 mol = 13.5 L / 0.000524 mol V 2 = (13.5 L / 0.000524 mol)(0.00144 mol) V 2 = 37.1 L
See another Avogadro’s law example problem .
- Avogadro, Amedeo (1810). “Essai d’une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons”. Journal de Physique . 73: 58–76. English translation
- Castka, Joseph F.; Metcalfe, H. Clark; Davis, Raymond E.; Williams, John E. (2002). Modern Chemistry . Holt, Rinehart and Winston. ISBN 978-0-03-056537-3.
- Scheidecker-Chevallier, Myriam (1997). “L’hypothèse d’Avogadro (1811) et d’Ampère (1814): la distinction atome/molécule et la théorie de la combinaison chimique”. Revue d’Histoire des Sciences (in French). 50 (1/2): 159–194. doi: 10.3406/rhs.1997.1277
Related Posts
What Is Avogadro’s Law (Avogadro’s Hypothesis Or Avogadro’s Principle)?
Avogadro’s law formula, avogadro’s number, moles to grams.
Avogadro’s law states that under conditions of constant pressure and temperature, there is a direct relationship between the number of moles and volume of a gas. This was Avogadro’s initial hypothesis. This law was applicable to ideal gases, while real gases show a slight deviation from it.
The modern definition of Avogadro’s law is that for a particular mass of an ideal gas, the amount (number of moles) and volume of the gas are directly proportional, provided the temperature and pressure conditions are constant.
Recommended Video for you:
Avogadro’s law’s mathematical formula can be written as:
V ∝ n or V/n = k
Where “V” is the volume of the gas, “n” is the amount of the gas (number of moles of the gas) and “k” is a constant for a given pressure and temperature.
Avogadro’s law formula describes how equal volumes of all gases contain the same number of molecules, under the same conditions of pressure and temperature. In other words, it describes that equal volumes of two different gases will have the same number of molecules as long as the temperature and pressure are the same.
Amadeo Avogadro was an Italian scientist of the 19 th century. He is known for making major contributions to chemistry, when it was just becoming a separate science field. His work came around the same as that of Jacques Charles (Charles Law), Robert Boyle (Boyle’s Law), etc. In fact, Avogadro’s Law, the hypothesis set by him, was among the laws on which the Ideal Gas Law is based.
An ideal gas can be defined as one in which the collisions between the molecules of the gas are elastic – i.e. there is no loss of kinetic or of momentum, and the molecules don’t have any intermolecular forces of attraction, i.e. they don’t have any interactions between them with the exceptions of the randomized collisions.
Before we get into understanding his work however, let us go over some basics.
A mole is a measure of the quantity of a substance. One mole of a substance is defined as that quantity which has as many units as the number of carbon atoms in 12 grams of C-12 carbon.
Another thing to remember is that a lot of these laws use STP or standard temperature and pressure. For STP, the value of temperature is 273.15 K (which is 0℃) while the value of pressure is 1atm or 760mmHg

Also Read: What Is Charles’s Law?
Avogadro’s number is the number of molecules of gas in a mole. This number is huge, the current figure being 6.022 x 10 23 . The unit for Avogadro’s number is mol -1 . This means that the measure of the entity in question is per mole. Avogadro’s number is usually symbolised by N.
It’s interesting to note that contrary to popular belief, Avogadro’s number was not discovered by Amedeo Avogadro. The concept of mole and the determination of the value of Avogadro’s number happened after Avogadro’s death. In fact, Avogadro’s number is so called in honour of his discovery and his work.

The first person to calculate the total number of particles present in a substance was an Austrian high school teacher called Josef Loschmidt, who, after a few years, became a professor at the University of Vienna.
Using kinetic molecular theory, Loschmidt was able to estimate the number of particles present in one cubic centimeter of gas at standard conditions of pressure and temperature. The value he calculated back in 1865 is known as the Loschmidt constant today, and its value is 2.6867773 x 1025 m-3.

The term ‘Avogadro’s number’ was first used by Jean Baptiste Perrin – a French physicist. He reported an estimate of the Avogadro’s number in 1909 based on his work on Brownian motion. For the uninitiated, Brownian motion is the random, haphazard movement of microscopic particles suspended in a gas/liquid.
Accurate determination of the Avogadro’s number only became possible for the first time when Robert Millikan – an American physicist – successfully measured the charge on an electron. Prior to this, the charge on a mole of electrons was already known (it’s a constant called ‘Faraday’, which is equal to 96,485.3383 coulombs per mole of electrons).
Certain parallels have been drawn to understand how humongous this number is. One of the easiest to comprehend is if this number of unpopped kernels of popcorn were spread across the area of the United States, after popping, the popcorn would cover the country up to a depth of 9 miles (For reference, the area of the United States is 3.797 square miles!!)
You will have noticed that I mentioned the current figure of Avogadro’s number. This is because over the years since the value was first determined, different methods have been used to calculate it. While each method gives approximately the same answer, there are slight variations. Therefore, based on the most recent calculations, that is the accepted figure. The first person to make this calculation, however, was Loschmidt.
Also Read: Grams To Moles: How To Convert Grams To Moles?
Moles can be converted to grams, which is another very popular measurement of quantity, and vice versa, by the following formula
Moles = grams/molar mass
To calculate the molar mass of a substance, one has to employ the use of the ever efficient periodic table. It be calculated by simply adding the mass number of the individual atoms in the substance. For instance, if one has to calculate the molar mass of NaCl –
Mass number of Na = 22.99 g/mol
Mass number of Cl = 35.45 g/mol
Therefore molar mass of NaCl is 22.99 + 35.45 = 58.44 g/mol
Avogadro’s number has a lot of applications in chemistry and physics. Certain generalization’s have also been drawn. For instance, the volume of 1 mole of a gas at STP is 22.4L. These are very handy in calculations. Avogadro’s number is an entity used by chemists worldwide. Although he may not have determined it, his work was the precedence for these calculations.
- Ideal Gas Law. The University of Oregon
- avogadro. The University of Kentucky
- Avogadro's Number. The University of California, Berkeley
- Moles to Grams Conversion Formula - Softschools.com. softschools.com

Mahak Jalan has a BSc degree in Zoology from Mumbai University in India. She loves animals, books and biology. She has a general assumption that everyone shares her enthusiasm about the human body! An introvert by nature, she finds solace in music and writing.
Gay-Lussac’s Law: How Does Pressure Of A Gas Vary With Its Temperature?
What Is Henry’s Law?
What Happens To A Gas When Its Pressure Is Increased?
What Is The Relationship Between Thermodynamics And Statistical Mechanics?

How Do You Weigh An Atom?

Without Technology, How Did We First Learn There’s No Oxygen In Space?

Can Metals Exist as 'Gases?'

Why Does Water Evaporate at Room Temperature?

Entropy : Why is it Predicted to Cause the Heat Death of the Universe?

Bose Einstein Condensate Explained in Simple Words

Why Is the Periodic Table Arranged the Way It Is?

Why Does Hydrogen Make That 'Pop' Sound Upon Burning?
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Avogadro’s Law
Avogadro’s law formula, applications and uses [1], problems and solutions.
Avogadro’s law states that equal volumes of different gases contain an equal number of molecules under the same pressure and temperature conditions. This law is valid for ideal gases at low pressures and high temperatures [1-4] .

Italian physicist Amedeo Avogadro was the first to state the hypothetical law in 1811.
According to Avogadro’s hypothesis, the volume (V) of a gas is directly proportional to the number of moles (n) [1-4] .
n : Number of moles
The above proportionality can be written as follows:
k : proportionality constant
This equation can be represented as a graph.

Consider a gas with n 1 moles with a volume V 1 . Suppose the number of moles increases to n 2 such that the volume increases to V 2 , then
V 1 = kn 1 and V 2 = kn 2
Dividing one equation by the other and rearranging,
V 1 /n 1 = V 2 /n 2
The above equation can be used to compare two gases at the same temperature and pressure conditions. Also, the number of molecules in a given volume of an ideal gas is independent of their size or the molar mass.

In order to derive Avogadro’s gas law, let us look at the ideal gas equation.
Or, V/n = RT/P
P : Pressure
R : Universal gas constant
T : Temperature
At constant pressure and temperature, the right-hand side is constant. Let, k = RT/P. Then,
Thus, the volume is proportional to the number of moles of the gas.
Avogadro’s Number
Let us rewrite the ideal gas law as follows:
PV = (N/N A )RT
The ratio N/N A gives the number of moles or n = N/N A . Here, N is the number of molecules in the gas, and N A is known as Avogadro’s number. It is the number of molecules present in one mole of a substance. Its value is 6.023 x 10 23 .
Molar Volume
Avogadro’s number can be used to calculate the molar volume. For one mole of a gas, n = 1 and V = k. Therefore, one has to know the value of k at standard temperature and pressure (STP). At STP, the pressure (P) is 101.325 kPa, and the temperature (T) is 273.15 K. The universal gas constant R has a value of 8.314 L·kPa·M -1· K -1 . Putting in all the values, we get
Or, k = 8.314 L·kPa·M -1· K -1 x 273.15 K /101.325 kPa
Or, k = 22.4 LM -1
Below are some examples of Avogadro’s law in everyday life [5] .
- Respiration : When we respire, we breathe in oxygen. The more we breathe, the more our lungs will expand.
- Deflation : When the air is released from an inflated tire, the number of moles decreases. The shape of the tire also changes since its volume decreases.
- Inflation : When a water tube is inflated by pumping air inside, the number of air molecules increases. Thus, the tube is inflated, and its volume increases.
- Explains Gay-Lussac’s law
- Determines the atomicity of the gases
- Determines the molecular formula of a gaseous compound
- Gives the relationship between gram molecular mass and gram molecular volume of gas
- Determines the relationship between molecular mass and vapor density of a gas
Problem 1 : Two moles of helium gas fill up an empty balloon to a volume of 2.5 L. What would be the volume of the balloon if an additional 1.5 moles of helium gas is added at constant temperature and pressure.
n 1 = 2 mol
V 1 = 2.5 L
n 2 = 2 mol + 1.5 mol = 3.5 mol
From Avogadro’s law,
Therefore, the final volume of the balloon is
V 2 = V 1 ·n 2 /n 1 = (2.5 L x 3.5 mol)/2 mol = 4.375 L
Problem 2 : 40 g of nitrogen gas is kept in a 2.5 L container. The gas exerts a pressure of 2 atm on the container. If pressure is kept constant, what is the final amount of gas in grams present in the container if gas is added until the volume has increased to 4.0 L?
Mass of nitrogen = 40 g => n 1 = Mass/Molar mass = 40 g/28 gM -1 = 1.43 M
Or, n 2 = n 1 x V 2 /V 1
Or, n 2 = 1.43 M x 4 L/2.5 L
Or, n 2 = 2.29 M
Therefore, amount of nitrogen
= 2.29 M x 28 gM -1 = 64 g
- Chem.libretexts.org
- Thoughtco.com
- Ch301.cm.utexas.edu
- Courses.lumenlearning.com
- Researchtweet.com
Related Articles

Sublimation

Graham’s Law

Crystal Field Theory

Van der Waals Equation
2 responses to “Avogadro’s Law”
Dat nice 🥰🤩
this is the best explanation of Avogadro’s law. I love this!!!!!!!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Trending Topics
© 2024 ( Chemistry Learner )
What Is Avogadro's Law? Definition and Example
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Avogadro's Law is the relation which states that at the same temperature and pressure , equal volumes of all gases contain the same number of molecules. The law was described by Italian chemist and physicist Amedeo Avogadro in 1811.
Avogadro's Law Equation
There are a few ways to write this gas law , which is a mathematical relation. It may be stated:
k = V/n
where k is a proportionality constant V is the volume of a gas, and n is the number of moles of a gas
Avogadro's law also means the ideal gas constant is the same value for all gases, so:
constant = p 1 V 1 /T 1 n 1 = P 2 V 2 /T 2 n 2
V 1 /n 1 = V 2 /n 2 V 1 n 2 = V 2 n 1
where p is pressure of a gas, V is volume, T is temperature , and n is number of moles
Implications of Avogadro's Law
There are a few important consequences of the law being true.
- The molar volume of all ideal gases at 0°C and 1 atm pressure is 22.4 liters.
- If pressure and temperature of a gas are constant, when the amount of gas increases, the volume increases.
- If pressure and temperature of a gas are constant, when the amount of gas decreases, the volume decreases.
- You prove Avogadro's Law every time you blow up a balloon.
Avogadro's Law Example
Say you have 5.00 L of a gas which contains 0.965 mol of molecules . What will be the new volume of the gas if the quantity is increased to 1.80 mol, assuming pressure and temperature are held constant?
Select the appropriate form of the law for the calculation. In this case, a good choice is:
V 1 n 2 = V 2 n 1
(5.00 L)(1.80 mol) = (x)(0.965 mol)
Rewriting to solve for x give you:
x = (5.00 L)(1.80 mol) / (0.965 mol)
x = 9.33 L
- Avogadro, Amedeo (1810). "Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons." Journal de Physique . 73: 58–76.
- Clapeyron, Émile (1834). "Mémoire sur la puissance motrice de la chaleur." Journal de l'École Polytechnique . XIV: 153–190.
- Chemistry Definition of Gas Constant (R)
- Avogadro's Law Example Problem
- Law of Combining Volumes Definition
- Avogadro's Number: Definition
- Law of Definite Proportions Definition
- The Combined Gas Law in Chemistry
- Oxidation Definition and Example in Chemistry
- What Is Electronegativity and How Does It Work?
- Partial Pressure Definition and Examples
- Entropy Definition in Science
- Theory Definition in Science
- Congener Definition and Examples
- Definition of the Law of Chemical Equilibrium
- Ideal Gas Law Definition and Equation
- Free Energy Definition in Science
- Single-Displacement Reaction Definition and Examples
February 16, 2004
How Was Avogadro’s Number Determined?
Chemist George M. Bodner of Purdue University explains
By George M. Bodner

Amadeo Avogadro.
Getty Images
Contrary to the beliefs of generations of chemistry students, Avogadro’s number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856). Avogadro was a lawyer who became interested in mathematics and physics, and in 1820 he became the first professor of physics in Italy. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles.
The first person to estimate the actual number of particles in a given amount of a substance was Josef Loschmidt, an Austrian high school teacher who later became a professor at the University of Vienna. In 1865 Loschmidt used kinetic molecular theory to estimate the number of particles in one cubic centimeter of gas at standard conditions. This quantity is now known as the Loschmidt constant, and the accepted value of this constant is 2.6867773 x 10 25 m -3 .
The term “Avogadro’s number” was first used by French physicist Jean Baptiste Perrin. In 1909 Perrin reported an estimate of Avogadro’s number based on his work on Brownian motion—the random movement of microscopic particles suspended in a liquid or gas. In the years since then, a variety of techniques have been used to estimate the magnitude of this fundamental constant.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Accurate determinations of Avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales using the same unit of measurement. This became possible for the first time when American physicist Robert Millikan measured the charge on an electron. The charge on a mole of electrons had been known for some time and is the constant called the Faraday. The best estimate of the value of a Faraday, according to the National Institute of Standards and Technology (NIST), is 96,485.3383 coulombs per mole of electrons. The best estimate of the charge on an electron based on modern experiments is 1.60217653 x 10 -19 coulombs per electron. If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadro’s number of 6.02214154 x 10 23 particles per mole.
Another approach to determining Avogadro’s number starts with careful measurements of the density of an ultrapure sample of a material on the macroscopic scale. The density of this material on the atomic scale is then measured by using x-ray diffraction techniques to determine the number of atoms per unit cell in the crystal and the distance between the equivalent points that define the unit cell (see Physical Review Letters, 1974, 33, 464).
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Chemistry
Course: ap®︎/college chemistry > unit 1.
- Average atomic mass
The mole and Avogadro's number
- Worked example: Calculating molar mass and number of moles
- Moles and molar mass
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Collections
- Sustainability in chemistry
- Simple rules
- Teacher well-being hub
- Women in chemistry
- Global science
- Escape room activities
- Decolonising chemistry teaching
- Teaching science skills
- Get the print issue
- RSC Education

- More navigation items
Amadeo Avogadro 1776-1856
By Bill Brock 2006-07-01T00:00:00+01:00
This year marks the 150th anniversary of the death of the Italian chemical physicist, Amedeo Avogadro.
This year marks the 150th anniversary of the death of the Italian chemical physicist, Amedeo Avogadro. Although commemorated for his molecular hypothesis, this was virtually ignored by chemists until after his death while his other research made no impact whatsoever. Avogadro's constant is, however, fundamental to molecular science.
When the American Chemical Society (ACS) celebrated its centenary in 1974 it issued car stickers that proclaimed: 'I know Avogadro's Number is 6.022 × 10 23 '. The implication was that, like C. P. Snow's claim for knowledge of the Second Law of Thermodynamics as a mark of good education, familiarity with Avogadro's number provides a similar test. The irony is that Avogadro never calculated the number and the eponym was only made 50 years after his death. The eponym, however, is justified on the grounds that Avogadro was one of the founders of modern molecular theory and of the standardisation of atomic weights.

Source: Neveshkin Nikolay/Shutterstock
Amadeo Avogadro
Avogadro's early days
Lorenzo Romano Amedeo Carlo Avogadro, the Count of Quaregna and Cerreto, was born in the Piedmontese city of Turin in the kingdom of Sardinia on 9 August 1776. He was the son of a prominent civil servant who was charged under the Napoleonic rule of 1799 to reorganise the Piedmont Government. The family had a long tradition of legal service to church and state - indeed, the surname Avogadro is a corruption of the Latin advocato , meaning a barrister.
Avogadro graduated in law from the University of Turin in 1795, and was awarded a doctorate in canon law the following year. He then followed in his father's footsteps by working for the Government, changing administrative roles after the French invaded Piedmont in 1799. Like Antoine Lavoisier, who had also studied law, Avogadro began to develop scientific interests in his spare time. He was particularly excited by Alessandro Volta's development of the electric battery in 1800. Indeed his interest in electricity prompted him to audit a course of lectures and demonstrations on physics at the University of Turin and to join the local Academy of Sciences. In 1804 he submitted his first two scientific papers to the Academy. The subjects of these papers, the nature of insulators (dielectrics) and the electromotive series, remained dominant interests of Avogadro throughout his life.
In 1806, determined to help reverse his country's perceived backwardness in the sciences, Avogadro abandoned his legal career and began to teach mathematics and physics at a succession of Piedmontese schools and colleges. In 1809, he was appointed professor of natural philosophy at the former Royal College in Vercelli, east of Turin. Living under a French political regime, he was attracted to French scientific culture and so began sending his scientific papers to Jean-Claude de Lamétherie's monthly Journal de physique.
What marked Avogadro from contemporary chemists was his ability to apply mathematics to physical observations in an attempt to explain chemical relationships between elements and compounds (acidities, affinities, atomic volumes and specific heats). However, his peers largely ignored his publications and, unlike his fellow countryman Volta, Avogadro was indifferent towards a reputation outside his own country, which he never left to meet other scientists. A theoretician in an age of experimentalists, and hindered by the political situation in Piedmont, Avogadro's work, which was often out of kilter with what was going on in the rest of European science, was destined for neglect in his lifetime.
Molecular hypothesis
According to historians, Avogadro proposed his molecular hypothesis to reconcile John Dalton's suggestion that atoms combine gravimetrically in the simplest possible way, as when single atoms of hydrogen and oxygen combine to form one compound atom of water:
H + O = HO
and Joseph Gay-Lussac's 1809 discovery of the integral law of combining volumes of gases:
2 vols H + 1 vol O = 2 vols water
(Berzelian symbols, which Avogadro did not adopt, are used for clarity.)
Gay-Lussac's experiments suggested that equal volumes of different gases ought to contain the same number of ponderable particles, provided that the physical conditions were constant. Yet, in practice, there was a glaring discrepancy between the expected ratio of volumes (in the case of water, 2:1:3) and the observed ratios (2:1:2). Moreover, the densities of the gaseous products were frequently at odds with those of the reactants. For example, in the case of the oxidation of carbon monoxide to form carbon dioxide:
100 vols CO + 50 vols O = 100 vols CO 2
the density of carbon dioxide (1.5196) was less than that of the half unit of oxygen (0.5518) that had gone into its formation. How could a gas that contained both carbon and oxygen be lighter than oxygen itself?
The gravimetric and volumetric information would make sense, Avogadro suggested in a French paper in 1811, if equal volumes of gases under the same physical conditions of temperature and pressure contained the same number of 'integral molecules', ie separable aggregates of at least two, but possibly more, particles. He was suggesting that both elementary and compound gaseous molecules divide at the moment of their reaction with other elementary and compound molecules to form the observed volumes of the products. For example, water was formed from a half-molecule of oxygen and two half-molecules of hydrogen.
2H 2 + O 2 = 2H 2 O
Because the numbers of such aggregated (or integral) particles were the same in equal volumes, it followed that the ratios of their densities (relative to a chosen standard density) stood in the same ratio as their relative masses. In the four papers Avogadro published on the subject between 1811 and 1821 he suggested how, on the basis of this hypothesis, vapour density measurements might be used to determine molecular weights, assuming that oxygen of molecular weight 16 was at least a dimer. Later, this gave the familiar formula: molecular weight = 2 × vapour density. There was never any implication that the particles that made up integral molecules were physical atoms and Avogadro probably did not believe in the existence of such indivisible entities. He did not therefore, as was assumed by later chemists, distinguish between atoms and molecules.
Avogadro's suggestion, however, was ignored or dismissed as fanciful speculation (though the Frenchman André-Marie Ampère did play with the notion in 1816). At the time the concept of two-identical atoms of an element combining to form a stable dimer was inconceivable (and, indeed, remained mysterious until the emergence of the notion of electron sharing in the early 1900s). According to the chemical authorities of the day, Dalton and Jacob Berzelius, like atoms were self-repulsive, because they were surrounded either by mutually repulsive atmospheres of heat (caloric) or of electricity. As an added twist, Avogadro's model had gas molecules surrounded by atmospheres of caloric, leading him to suppose they must be the same size in all gases.
A further difficulty was that Avogadro could only determine the vapour densities of a handful of gases, and his theoretical calculations of the vapour densities of solid elements worked to four or five decimal places, based upon the weight of the element that combined with 16 parts (one volume) of oxygen, seemed entirely hypothetical. Finally, readers of his papers were probably confused by his cumbersome usage of the terms molecule (either an atom or molecule), integral molecule (usually the molecule of a compound), constituent molecule (one molecule of the element) and elementary molecule (one atom). In fact this molecular vocabulary was unique to French writers at the end of the 18th century and provides historians with the clue to understanding Avogadro's research programme.
Support for Berthollet
The geographically isolated Avogadro belonged intellectually to the French research school of Lavoisier's colleague Simon Laplace, the mathematician and astronomer, and Lavoisier's chemistry disciple, Claude Berthollet. 1 In his famous dispute with analytical chemist, Louis-Joseph Proust at the end of the 18th century Berthollet had insisted that constant composition was limited in application. Chemical composition was determined by physical conditions and concentration, since these affected the natural affinities between elements. Although Dalton had built the laws of constant and multiple composition into his atomic theory at the beginning of the 19th century, Berthollet's version of the Law of Mass Action (as it became known in the 1870s) intrigued Avogadro who spent much of his life trying to determine absolute affinity figures from physical data in support of the French programme. Gases were of a primary interest to him (as they were to Berthollet's pupil Gay-Lussac) because, unlike solids and liquids, their affinities were completely saturated.
Unfortunately, after a promising start, Berthollet's research programme, which involved the caloric theory as well as Laplace's idea of short range forces of attraction and repulsion, led nowhere and was abandoned by French scientists after about 1815. Avogadro, however, kept the faith; consequently his later work failed to enhance his stature among European chemists. 2 His multi-volume physics textbook, Fisica de' corpi ponderabili , published when he was in his sixties between 1837 and 1841, showed him to have been left behind completely by the advances in physics in other countries.
Avogadro remained at Vercelli until 1820 when he returned to the University of Turin as its first professor of mathematical physics only to lose it a year later when political upheaval caused the chair to be suspended. During the next 10 years he returned to practice law, but was reappointed to the re-established Turin chair in 1834. In 1850 he and his wife retired to their family estates at Quaregna. He died on a visit to Turin 150 years ago on 9 July 1856. None of his obituaries mentioned the molecular hypothesis, though they described him as 'religious without intolerance, learned without pedantry, wise without ostentation, a despiser of pomp, without care for riches, not ambitious for honours; ignorant of his own worth and fame, modest, temperate, and lovable'. 3
Although warmly regarded by the Piedemontese as a man of great learning and modesty, Avogadro had been little known outside the Italian peninsular during his lifetime, despite his French offerings. His career supports the views of sociologists of science who stress that historical, social, political and cultural factors play important roles in the emergence of scientific knowledge. 4 Avogadro sat at the edge of the French scientific empire but lacked any personal contact with French (let alone English and Scandinavian) chemists. The result was intellectual isolation and invisibility.
Avogadro honoured
This changed with the Risorgimento that culminated in the unification of Italy in 1870. Italians wanted to underwrite their new nationhood by looking for cultural heroes. Posthumously Avogadro became seen as one of the founders of molecular theory after Stanislao Cannizzaro showed, in 1858, and more forcibly at the Karlsruhe Congress of chemists two years later, how a rational and standardised system of atomic weights could be achieved by distinguishing between atoms and molecules. Cannizzaro put Avogadro's hypothesis at the forefront of chemical theory. The different quantities of the same element contained in one molecule of the free substance and in those of its compounds were then always integral multiples of a quantity that represented the atomic weight. This standardisation removed much confusion and complemented the views of contemporary organic chemists. 5
By the 1870s the kinetic theory of gases had given credence to the atomic-molecular theory and it was the Bohemian chemist Josef Loschmidt (1821-95) who used the gas laws to calculate the number of molecules in a standard volume, the cubic centimetre. His value of 2.6 × 10 19 has since been recalculated as 2.68 × 10 25 m -3 . This is still known as Loschmidt's number. However, when Jean Perrin used Brownian motion to provide convincing proof of the existence of molecules in 1909 he used the gram molecule (mole) as his standard and calculated the value as 6 × 10 23 . Today this is defined as the number of molecules in 12 grams of the carbon-12 isotope. It was Perrin who suggested that this important chemical constant should be designated as Avogadro's number in commemoration of Avogadro's original insight in 1811.
Bill Bryson notes in A short history of nearly everything , American College students in the 20th century estimated that the stupendous figure of 6 × 10 23 was equivalent to the number of popcorn kernels needed to cover the US to a depth of nine miles. Have Italian students a pizza version?
Bill Brock is emeritus professor of history of science at the University of Leicester, University Road, Leicester LE1 7RH.
Further Reading
- Gayle Brickert-Albrecht and Dan Morton, A biographical interview with Avogadro .
- N. G. Coley, The physico-chemical studies of Amedeo Avogadro , Ann. Sci ., 1964, 20 , 195-210.
- Nicholas Fisher, Avogadro, the chemists, and historians of chemistry , Hist. Sci ., 1982, 20 (77), 212.
- Mario Morselli, Amedeo Avogadro . Dordrecht: D. Reidel, 1984.
- Maurice Crosland, The Society of Arcueil: a view of French science at the time of Napoleon I . London: Heinemann, 1967.
- Robert Fox, The caloric theory of gases. From Lavoisier to Regnault , Chap 6. Oxford: Clarendon, 1971.
- Eric J. Holmyard, Makers of chemistry , p 250. Oxford: Clarendon, 1931.
- Jan Golinski, Making natural knowledge. Constructivism and the history of science . Cambridge: CUP, 1998.
- Alan J. Rocke, The quiet revolution: Hermann Kolbe and the science of organic chemistry . Berkeley: University of California, 1993.
Related articles

Calculating moles and using Avogadro’s number
2022-05-03T09:55:00Z By Emily Rose Seeber
Use this explainer to help your students grasp the relationship between moles, mass and molecular mass
1 Reader's comment
Only registered users can comment on this article., more feature.

Enhance students’ learning and development with digital resources
2024-06-17T05:02:00Z By David Paterson
Tips and a model to improve learners’ understanding and develop vital skills using digital learning

Getting the most out of the UK Chemistry Olympiad
2024-06-05T07:00:00Z
It’s the competition with something for every learner and teacher. Discover the benefits of participation here

Your guide to the UK Chemistry Olympiad
2024-06-05T07:00:00Z By Nina Notman
Discover how your school can easily participate in the leading annual chemistry competition for secondary school learners
- Contributors
- Print issue
- Email alerts
Site powered by Webvision Cloud

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
Education and early career
Molecular hypothesis of combining gases, family and legacy.

Amedeo Avogadro
Our editors will review what you’ve submitted and determine whether to revise the article.
- Brooklyn College - Biography of Amedeo Avogadro
- Linda Hall Library - Scientist of the Day - Amedeo Avogadro
- Famous Scientists - Biography of Amedeo Avogadro
- Science History Institute - Amedeo Avogadro
- Amedeo Avogadro - Student Encyclopedia (Ages 11 and up)
- Table Of Contents

Amedeo Avogadro (born August 9, 1776, Turin , in the Kingdom of Sardinia and Piedmont [Italy]—died July 9, 1856, Turin) was an Italian mathematical physicist who showed in what became known as Avogadro’s law that, under controlled conditions of temperature and pressure , equal volumes of gases contain an equal number of molecules .
Avogadro was the son of Filippo Avogadro, conte di Quaregna e Cerreto, a distinguished lawyer and senator in the Piedmont region of northern Italy . Avogadro graduated in jurisprudence in 1792 but did not practice law until after receiving his doctorate in ecclesiastical law four years later. In 1801 he became secretary to the prefecture of Eridano.

Beginning in 1800 Avogadro privately pursued studies in mathematics and physics , and he focused his early research on electricity . In 1804 he became a corresponding member of the Academy of Sciences of Turin, and in 1806 he was appointed to the position of demonstrator at the academy’s college. Three years later he became professor of natural philosophy at the Royal College of Vercelli, a post he held until 1820 when he accepted the first chair of mathematical physics at the University of Turin . Due to civil disturbances in the Piedmont, the university was closed and Avogadro lost his chair in July 1822. The chair was reestablished in 1832 and offered to the French mathematical physicist Augustin-Louis Cauchy . A year later Cauchy left for Prague, and on November 28, 1834, Avogadro was reappointed.
Avogadro is chiefly remembered for his molecular hypothesis , first stated in 1811, in which he claimed that equal volumes of all gases at the same temperature and pressure contain the same number of molecules . He used this hypothesis further to explain the French chemist Joseph-Louis Gay-Lussac ’s law of combining volumes of gases (1808) by assuming that the fundamental units of elementary gases may actually divide during chemical reactions . It also allowed for the calculation of the molecular weights of gases relative to some chosen standard. Avogadro and his contemporaries typically used the density of hydrogen gas as the standard for comparison. Thus, the following relationship was shown to exist: Weight of 1 volume of gas or vapour / Weight of 1 volume of hydrogen = Weight of 1 molecule of gas or vapour / Weight of 1 molecule of hydrogen
To distinguish between atoms and molecules of different kinds, Avogadro adopted terms including molécule intégrante (the molecule of a compound ), molécule constituante (the molecule of an element ), and molécule élémentaire (atom). Although his gaseous elementary molecules were predominantly diatomic, he also recognized the existence of monatomic, triatomic, and tetratomic elementary molecules. In 1811 he provided the correct molecular formula for water, nitric and nitrous oxides , ammonia , carbon monoxide , and hydrogen chloride . Three years later he described the formulas for carbon dioxide , carbon disulfide , sulfur dioxide , and hydrogen sulfide . He also applied his hypothesis to metals and assigned atomic weights to 17 metallic elements based upon analyses of particular compounds that they formed. However, his references to gaz métalliques may have actually delayed chemists’ acceptance of his ideas. In 1821 he offered the correct formula for alcohol (C 2 H 6 O) and for ether (C 4 H 10 O).
Priority over who actually introduced the molecular hypothesis of gases was disputed throughout much of the 19th century. Avogadro’s claim rested primarily upon his repeated statements and applications. Others attributed this hypothesis to the French natural philosopher André-Marie Ampère , who published a similar idea in 1814. Many factors account for the fact that Avogadro’s hypothesis was generally ignored until after his death. First, the distinction between atoms and molecules was not generally understood. Furthermore, as similar atoms were thought to repel one another, the existence of polyatomic elementary molecules seemed unlikely. Avogadro also mathematically represented his findings in ways more familiar to physicists than to chemists. Consider, for example, his proposed relationship between the specific heat of a compound gas and its chemical constituents: c 2 = p 1 c 1 2 + p 2 c 2 2 + etc.
(Here c , c 1 , c 2 , etc., represent the specific heats at constant volume of the compound gas and its constituents; p 1 , p 2 , etc., represent the numbers of molecules of each component in the reaction). Based upon experimental evidence, Avogadro determined that the specific heat of a gas at constant volume was proportional to the square root of its attractive power for heat. In 1824 he calculated the “true affinity for heat” of a gas by dividing the square of its specific heat by its density. The results ranged from 0.8595 for oxygen to 10.2672 for hydrogen, and the numerical order of the affinities coincided with the electrochemical series, which listed the elements in the order of their chemical reactivities. Mathematically dividing an element’s affinity for heat by that of his selected standard, oxygen, resulted in what he termed the element’s “ affinity number.” Between 1843 and his retirement in 1850, Avogadro wrote four memoirs on atomic volumes and designated affinity numbers for the elements using atomic volumes according to a method “independent of all chemical considerations”—a claim that held little appeal for chemists.

Avogadro married Felicita Mazzé of Biella in 1815; together they had six children. Home-loving, industrious , and modest, he rarely left Turin. His minimal contact with prominent scientists and his habit of citing his own results increased his isolation. Although he argued in 1845 that his molecular hypothesis for determining atomic weights was widely accepted, considerable confusion still existed over the concept of atomic weights at that time. Avogadro’s hypothesis began to gain broad appeal among chemists only after his compatriot and fellow scientist Stanislao Cannizzaro demonstrated its value in 1858, two years after Avogadro’s death. Many of Avogadro’s pioneering ideas and methods anticipated later developments in physical chemistry . His hypothesis is now regarded as a law , and the value known as Avogadro’s number (6.02214076 × 10 23 ), the number of molecules in a gram molecule, or mole , of any substance, has become a fundamental constant of physical science .
- Avogadro’s Law
According to Avogadro’s Law, all gases have an identical number of molecules in an equal volume at a given temperature and pressure. Amedeo Avogadro, an Italian chemist, and physicist, first described the law in 1811. Amadeo Avogadro was a scientist from Italy in the 1800s. When chemistry was just starting to become its science field, he made important contributions to it. His work was done around the same time as that of Jacques Charles, Robert Boyle, and others. The Ideal Gas Law is based in part on Avogadro’s Law, which is a hypothesis he came up with.

Avogadro’s Law
What is Avogadro’s Law?
The quantity (number of moles) and volume of an ideal gas are directly proportional to each other for a given mass of the gas at constant temperature and pressure , according to the modern definition of Avogadro’s law. Avogadro’s law connects temperature, pressure, volume, and substance amount for a certain gas, which makes it closely related to the ideal gas equation. The behaviour of particles in an ideal gas, which lacks mass and is not attracted to one another, can be explained by the collisions between gas molecules and the container’s walls.
Real gases do not, of course, exist in an ideal state, but since they are so little and are surrounded by so much space, it is difficult to estimate their size and mass, which is why it is not important. As a result, under most circumstances, most gases behave quite “ideally.”
Avogadro’s Law Formula
According to Avogadro’s rule, a gas’s volume, V, is inversely related to its particle count, n. This link can be described mathematically as follows:
Mole counts are used by chemists to determine the number of atoms and molecules. The quantity of particles that make up a mole of a substance is known as Avogadro’s number or NA. It has been established through numerous investigations that NA has a particle density per mole of 6.02 × 1023.
In other words, the volume V to the number of gas particles n ratio equals a proportionality constant k.
\(\frac{V}{n}\) = k
\(\frac{V1} {n1}\) =\(\frac{V2} {n2}\)
This equation says that when the number of particles in a gas changes from n1 to n2, the volume also changes from V1 to V2.
Derivation of Avogadro’s Law
The ideal gas equation, which can be written as follows, can be used to figure out Avogadro’s law:
P= the pressure that the gas puts on the walls of its container
V= volume that the gas did take up
n=number of moles of gas
R= gas constant
T= absolute temperature
If you rearrange the equation for an ideal gas, you will get the following equation:
\(\frac{V}{n}\) = \(\frac{RT}{P}\)
The value of RHS (Right Hand Side) is constant. Then,
\(\frac{v}{n}\) = k
So, the relationship between the amount of space a gas takes up and the number of molecules in the gas is proven.
Graphical Representation

There is a straight-line relationship between the volume of a gas and the number of moles of gas particles. At the same temperature and pressure, as the volume of gas goes up, so does the number of moles of gas.
Moles to Grams
The following formula shows how to change from moles to g, which is another common unit of measure:
Moles = \(\frac{grams}{molar mass}\)
To figure out what a substance’s molar mass is, you have to use the useful periodic table. It can be worked out by adding up the masses of all the atoms in the substance. For example, if you need to figure out the molar mass of NaCl, you would:
Na has a mass number of 22.99 g/mol.
Cl’s mass number is 35.45 g/mol.
So, the molar mass of NaCl is 22.99 plus 35.45, which equals 58.44 g/mol.
Molar Volume of a Gas
The formula for the ideal gas law, PV = nRT, can be used to find the molar volume, or V, of a gas. In this equation, P is the pressure, n is 1 mol, R is the universal gas constant, and T is the temperature in Kelvin. The value of R will change depending on the pressure and volume units that are used.
Examples of Avogadros Law
A great example of Avogadro’s law is the way that we breathe. When a person breathes in, the molar amount of air in their lungs goes up, and so does the volume of their lungs (expansion of the lungs). The way car tires lose air is another common example of Avogadro’s law. When the air that was trapped in the tire gets out, the amount of air in the tire goes down. This causes the gas to take up less space, which causes the tire to lose its shape and deflate.
What are the Limitations of Avogadro’s Law?
Even though it works perfectly for all ideal gases, Avogadro’s law only tells us how the real gases relate to each other. The difference between how real gases behave and how they should behave tends to get bigger as pressure and temperature go up. Hydrogen and helium, which are gases with low molecular masses, follow Avogadro’s law better than molecules with higher molecular masses.
Solved Exercises on Avogadro’s Law
Question 1. At 25°C and 2.00 atm, a sample of 6.0 L holds 0.5 moles of a gas. What is the final volume of the gas if 0.25 moles more are added at the same pressure and temperature?
Solution. Avogadro’s law:
\(\frac{V1}{n1}\) = \(\frac{V2}{n2}\)
V1= initial volume = 6.0 L
n1= initial number of moles = 0.5 mole
V2= final volume = x L
n2= final number of moles= 0.5 + 0.25 = 0.75 mole
\(\frac{6.0}{0.5}\) = \(\frac{x}{0.75}\)
x = \(\frac{6.0 × 0.75}{0.5}\)
Question 2. A puncture takes away half of the volume of a tire with 10 moles of air and a 40-litre volume. How much air is left in a tire that has been deflated?
\(\frac{V1}{n1}\)= \(\frac{V2}{n2}\)
V1= initial volume = 40 L
n1= initial number of moles = 10 mole
V2= final volume = 20 L
n2= final number of moles= x mole
\(\frac{40}{10}\) = \(\frac{20}{x}\)
x= \(\frac{20 × 10}{40}\)
FAQs on A vogadro’s Law
Question 1. What does the law of Charles law say?
Answer. Avogadro’s law looks at the relationship between the amount of gas (n) and the amount of space it takes up (V). It’s a direct relationship, which means that the amount of moles in a gas is proportional to how much space it takes up.
Question 2. Explain Avogadro’s idea?
Answer. Avogadro’s law says that when the temperature and pressure are the same, the same number of molecules are found in equal volumes of different gases. Under the assumption of an ideal gas, the kinetic theory of gases can be used to figure out this empirical relationship.
Customize your course in 30 seconds
Which class are you in.

- Difference between Ideal Gas and Real Gas
- Planck’s Quantum Theory
- Types of Minerals
- Isomers of Butane
- Saturated Hydrocarbons
- Endothermic Reaction
- Lewis Dot Structures
- Standard Electrode Potential
- Order of Reaction

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Talk to our experts
1800-120-456-456
- Avogadro Law

What is Avogadro's Law?
Avogadro’s law is a gas law and is also referred to as Avogadro’s hypothesis or Avogadro’s principle. It states that the total number of molecules or atoms of a gas is directly proportional to the volume that the gas occupies at a constant temperature and pressure. This law is much related to the ideal equation of the gas because it links the temperature, volume, pressure and the amount of substance for a gas.
This law is named after Amedeo Carlo Avogadro, an Italian scientist, who had suggested that two different gases that occupy the same amount of volume at constant pressure and temperature must have equal numbers of atoms or molecules.
In this article, you would the notes for Avogadro law class 11 which includes what is Avogadro’s law, Avogadro's law definition, Avogadro's hypothesis, state Avogadro’s law, Avogadro's law formula, and Avogadro's law example.
Avogadro's Law Formula
At constant pressure and temperature, the Avogadro’s law is expressed as follows:
\[\frac{V}{n}\] = k
Here, V is the volume of the gas
n is the amount of gaseous substance expressed in moles, and
k is constant
When the amount of the gaseous substance is allowed to increase, the increase in the volume that is occupied by the gas is calculated as follows:
\[\frac{V_1}{n_1}\] = \[\frac{V_2}{n_2}\] = k , according to Avogadro’s law
It is graphically represented as follows.
(Image will be uploaded soon)
Here, the straight line that indicates that both the quantities are directly proportional tends to pass through the origin, which implies that zero moles of gas occupy zero volume.
Derivation of Avogadro’s Law
Avogadro’s law is derived from the ideal gas equation, which is expressed as follows:
P refers to the pressure exerted by a gas on its container wall
V refers to the volume occupied by gas
n is the total amount of gaseous substance or number of moles of the gas
R refers to the universal gas constant, and
T refers to the absolute temperature of the gas
After rearranging the ideal gas equation,
\[\frac{V}{n}\] = \[\frac{(RT)}{P}\] in which,
the value of \[\frac{(RT)}{P}\] is constant because the temperature and pressure are kept constant and the product of two or more constants is a constant. Hence,
Molar Volume of a Gas
According to Avogadro’s law, the ratio of the volume and amount of gaseous substance is constant at constant pressure and temperature and is denoted by k. This constant is determined by
k = \[\frac{(RT)}{P}\]
Under standard temperature and pressure,
T = 273.15 K and P = 101.325 kiloPascals
Hence, the volume of one-mole gas at STP is,
\[\frac{(8.314 J.mol-1.K-1)(273.15 K)}{(101.325 kPa)}\] = 22.4 litres
Hence, one mole of any gas occupies 22.4 litres volume at STP.
Avogadro’s Law Example
The respiration process is an example of Avogadro’s law. When we inhale, there is an increase in the molar quantity of the air in our lungs. This leads to an increase in the lungs volume. Take a look at the image below.
Limitations of Avogadro’s Law
Even though it is perfectly applicable to all the ideal gases, Avogadro’s law only provides the relationships for the real gases. The deviation of the real gases from the ideal behaviour tends to increase at higher pressures and lower temperatures. Gaseous molecules that have lower molecular masses like hydrogen and helium follow Avogadro’s law to a good extent when compared to the heavier molecules.
Solved Example on Avogadro’s Law
Example 1: A tyre that consists of 10 moles air and occupies 40L volume loses half of the volume because of a puncture. What is the amount of air left in the deflated tyre at STP?
Solution:
Initial amount of air n 1 is 10 mol
Initial volume of tyre V 1 is 40 L
Final volume of tyre V 2 is 20 L
According to Avogadro’s law,
the amount of air left in the deflated tyre,
n 2 = \[\frac{V_{2}n_{1}}{V_1}\] = 5 moles
Hence, the deflated tyre contains 5 moles air.
Important Points to Remember
Equal volumes of various gases have an equal number of molecules under similar conditions of temperature and pressure.
Avogadro law is only for gas.
Avogadro law is important because the amount of gas and volume is detected by using this law.
An example of the Avogadro law is the deflation of automobile tyres.
The value of the constant can be determined with the help of an equation k = (RT)/P
Experts use the Avogadro law more in Chemistry to measure the pressure or the number of gases that exist in the vessel.
This is also used by the chemical and the process engineers for taking the calculations.
Gases with molecules that have less low molecular mass like helium and hydrogen obey the Avogadro law than the gases with higher molecular masses.
The process of respiration is another example of Avogadro law.
When the temperature and pressure are the same or constant then the amount increases then the volume increases.
When the temperature and pressure are the same or constant then the amount decreases then the volume also decreases.
Avogadro law is named after a famous Italian scientist Amedeo Carlo Avogadro.
Tips to secure Better Marks in the Exams regarding This Subject
Pay Attention - Students should pay attention in the classes where the chapter is covered by the teacher and try to make notes while listening to the teacher.
Read the Chapter - After the chapter is covered read the chapter and try to understand the topic while referring to the notes made during the class.
Clear Your Basics - Physics is all about the basics and these should be cleared from the beginning like from earlier classes so that they won't face problems when they reach Class 10,11 and 12. Always keep your basics strong so that the students can understand the topics which will be taught in the future because those concepts will help them to understand the other chapters.
Memorize the Basics - here the basics means the formulas or equations which are in physics because there are problems that have to be solved by applying the formulas and in order to do so students need to know which formula to be used to solve the questions. Some students mix up the formulas in different problems; this leads to having the wrong solutions. Equations are important to solve the practicals so it has to be memorized.
Derivation of Formulas - students should also keep in mind how the equation is derived because that will help them to understand why the equation will work in the problem which will give a step to have the right answer to a problem.
Attend the Problems in the Exam - Some students leave the questions which have problems in it but they fail to understand that that is the best way to score marks in exams because the marks will be given step-wise if the student has answered it accordingly. In order to achieve this one has to practice the problems when they have time.
Practice Makes Perfect - In Physics solving the problems is important so students should not avoid practicing the problems from the exercises given in the book and they should also practice the examples given in the book on their own this will make them understand the solutions. They will know why the equations are used in a question.
Doubts Clearing - Always try to be curious to know more, this will create doubts in the mind regarding any topic and write it down in the notes they can ask the teachers, by this students get to learn many new things in the process.
Make Notes - Always try to make notes by referring to the book or the study materials they have, sometimes they get extra information which can be useful in exams.
Extra Guidance - Physics is a subject where students are recommended to have extra guidance to study like taking extra tuitions for this subject because some students understand more easily and they also clear their doubts with their tutors.
Double-Check the Answers - Always try to solve the questions with the proper understanding of the problem and after that double-check the answers which are given in the solution books. Students can download the solutions books from the Vedantu website.
Vedantu has the facility for online tuitions for Physics and they can enroll for the tuitions, they will be provided with the best teachers of the subjects they want, they will be provided with the study materials so that they won't have to worry about the notes.

FAQs on Avogadro Law
1. State and explain Avogadro’s law.
According to Avogadro’s law, equal volumes of gases at constant pressure and temperature tend to occupy the same number of molecules. If the mass of the ideal gas is given, the volume and the number of moles present in the gas are proportional directly at a constant temperature and pressure. This relation is derived from the kinetic theory of the gases when there is an assumption of an ideal gas. The Avogadro’s law is valid approximately for the real gases at sufficiently higher temperatures and lower pressures.
2. State Avogadro’s hypothesis.
According to Avogadro's hypothesis, two samples of the gas having equal volume, at a constant temperature and pressure, consist of the same number of gaseous molecules. With the help of Avogadro's hypothesis, chemists are able to predict the behaviour of the ideal gasses. Amedeo Avogadro derived the hypothesis in the year 1811. Elaborating on the hypothesis, he quoted that the volume of the given gas does not tend to depend on the size of the mass of the molecules of the particular gas. Avogadro's hypothesis was then applied in the Avogadro's law for denoting that the volume of a gas, V is equal to the constant, k, multiplied by the number of the moles of the gas, which is given by, V=kn.

Avogadro’s Hypothesis: Law: Example: Application
- August 7, 2022
- Gaseous State
Table of Contents
Avogadro’s Hypothesis , introduced by Amaedo Avogardro in 1811, explains that in every gas the number of molecules is proportional to the volume. He replaced the term ‘atoms’ in the Berzelius hypothesis with the term ‘molecules’ and postulated a law, popularly known as Avogadro’s hypothesis.
Avogadro’s hypothesis states, “ Equal volumes of all gases and vapors contain an equal number of molecules under similar conditions of temperature and pressure .” According to this hypothesis, under the same temperature and pressure, the volume of a gas depends upon the number of molecules or moles but not upon their size or mass. Under the assumption of an ideal (perfect) gas , this empirical relationship can be derived from the kinetic theory of gases .
This law is valid for real gases at low pressure and high temperature.
Example of Avogadro’s law
If we consider 2 liters of nitrogen, carbon dioxide, and hydrogen each under STP, they will contain an equal number of molecules however their mass is different. This hypothesis is experimentally verified and is called Avogadro’s law . Avogadro’s law can explain gaseous reactions without violating Dalton’s atomic theory .

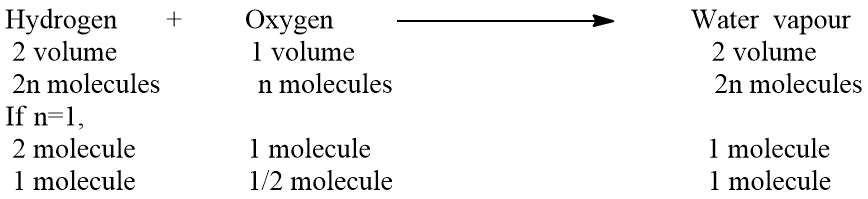
Let 1 volume of gas contain ‘n’ molecule then apply Avogadro’s law

The above experimental result shows that 1 molecule of hydrogen chloride is produced from 1/2 molecule of hydrogen and half molecule of chlorine. The fraction of molecules is possible because a molecule contains more than one atom . Hence Avogadro’s hypothesis is in accordance with dalton’s atomic theory and Gay-Lussac’s law of gaseous volume.
Application of Avogadro’s law
Avogadro’s law has numerous applications, some of which are as follows:
1. Determination of atomicity of elementary gases
Atomicity is the number of atoms present in one molecule of an element or compound. elementary gases are composed of atoms of the same element. For example, H 2 , Cl 2 , O 2 , N 2 , etc. Other types of gases are compound gases like CO 2 , NH 3 , SO 2 , N 2 O 5 , etc. Avogadro’s law is applied to determine the atomicity of elementary gases. Elementary gases are diatomic, i.e., one molecule contains two atoms. For Further illustration, we can take the example of oxygen.
1.1.Oxygen is Diatomic gas
Oxygen reacts with hydrogen to give water vapor. Experimentally, it has been found that hydrogen and oxygen react in the ratio of 2:1 by volume to give 2 volumes of water vapor under similar conditions of temperature and pressure. This experimental result is formulated as follows:
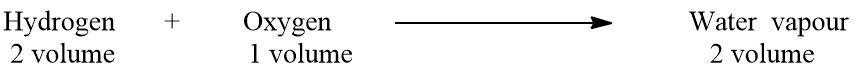
Let one volume of gas contain ‘n’ molecules, then by applying Avogadro’s hypothesis,
One molecule of water vapor contains one molecule of hydrogen and 1/2 molecule of oxygen. From Avogadro’s law , it has been proved that one molecule of hydrogen contains 2 atoms. The valency of hydrogen is one. Hence, two atoms of hydrogen can satisfy the valency of only one atom of oxygen because the valency of oxygen is two. From the above discussion, we come to know that one molecule of water vapor contains only one atom of oxygen. This one atom is derived from its half molecule. Hence, we can write,
1/2 molecule of oxygen contains 1 atom or 1 atom of oxygen contains 2 atoms.
Hence, by applying Avogadro’s hypothesis we are able to determine the atomicity of oxygen i.e, 2.
2. Deduction of the relationship between molecular mass and vapor density
Molecular mass or molecular weight is the mass of one molecule of a substance as compared to the mass of one atom of hydrogen.
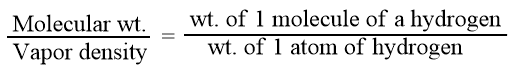
From Avogadro’s law, we know that hydrogen is diatomic i.e. a molecule containing two atoms. Hence, we can write
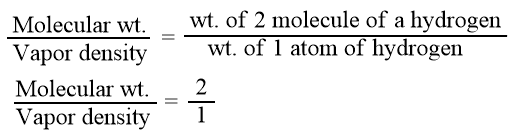
Mol. wt. = 2 × V.D.
Hence, the molecular weight of a gaseous substance is twice its vapor density .
3. Derivation of the relationship between gram molecular mass and volume of gas
The molecular weight expressed in grams is called gram molecular mass. Experimentally, it has been found that the gram molecular mass of any gas or vapor at STP occupies 22.4 liters. This experimental observation is theoretically explained by applying Avogadro’s law .
4. Derivation of the relationship between gram molecular weight and number of molecules
From Avogadro’s law, we know that the gram molecular weight of any gas or vapor occupies 22.4 liters at STP. This quantity is also called molar volume i.e, the volume occupied by one molecule. According to Avogadro’s law, we know that the equal volume of all gases and vapors contains an equal number of molecules under similar conditions of temperature and pressure.
Since one mole of any gas or vapor at STP occupies 22.4 liters. Due to equal volume, it must contain the same number of molecules. Experimentally it has been found that 22.4 liters of gas at STP contain 6.023 × 10 23 molecules.
5. Determination of molecular formula from volume composition of a gas
Avogadro’s law is also applied to determine the molecular formula of a gaseous compound from its volume composition and molecular mass. It can be explained by considering an oxide of nitrogen having a molecular mass of 60 and one volume of which is composed of half of the volume of nitrogen and half of the volume of oxygen i.e. volume ratio of nitrogen: oxygen: nitrogen oxides is 1:1:2 under the similar condition of temperature and pressure.
What is Avogadro’s hypothesis?
Avogadro’s hypothesis states, “ Equal volumes of all gases and vapors contain an equal number of molecules under similar conditions of temperature and pressure .”
What evidence supports Avogadro’s hypothesis?
The fraction of molecules is possible because a molecule contains more than one atom.
Share this to:
- Tags: according to avogadro's hypothesis , application of avogadro's hypothesis , avogadro's even hypothesis , avogadro's hypothesis and diatomic molecules , avogadro's hypothesis applications , avogadro's hypothesis chemistry , avogadro's hypothesis definition , avogadro's hypothesis definition chem , avogadro's hypothesis equation , avogadro's hypothesis example , avogadro's hypothesis explanation , avogadro's hypothesis formula , avogadro's hypothesis lab , avogadro's hypothesis questions , avogadro's hypothesis states that , avogadro's hypothesis states that: , avogadro's hypothesis worksheet answers , state avogadro's hypothesis , state avogadro's hypothesis. , what evidence supports avogadro's hypothesis
You may also like to read:

Evaporation: Definition, Process, and 5 Reliable Example

Cationic Polymerization: An Easy Mechanism and Kinetics

Corrosion: Definition, Cause, Types, Control, and 7 Importance

Polymorphism and Isomorphism: Definition and 5 Reliable Differences

Bronsted Bjerrum equation and Kinetic salt effect

Diffusion Controlled Reaction: Easy Definition, Kinetics
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Top Universities in the USA for Undergraduate and Graduate Chemistry Courses
Preparation of Phthalimide from Phthalic acid by two-step synthesis: Useful Lab Report
Feel Good Hormones: Dopamine, Oxytocin, Serotonin, and Endorphin
Invisible ink: Chemistry, Properties, and 3 Reliable Application
Pyrrole Disorder: Symptoms, Causes, and Treatment
The Chemistry of Mehendi: Composition, Side effects, and Reliable Application
How to Balance Redox Equations Using a Redox Reaction Calculator
Sugar Vs Jaggery: Differences, Calories And Many more
Centrifugation: Definition, Principle, Types, and 3 Reliable Application
Eppendorf Tube: Definition, Types, and Reliable Uses
Litmus Paper: Definition, Chemistry, Test, and 4 important Applications
Our mission is to provide free, world-class Chemisry Notes to Students, anywhere in the world.
Chemist Notes | Chemistry Notes for All | 2024 - Copyright©️ ChemistNotes.com

Make Waves in Learning! 25% off
for World Oceans Day
Use code OCEAN25

Share this article

Table of Contents
Latest updates.

1 Million Means: 1 Million in Rupees, Lakhs and Crores

Ways To Improve Learning Outcomes: Learn Tips & Tricks

Visual Learning Style for Students: Pros and Cons

NCERT Books for Class 6 Social Science 2024 – Download PDF

CBSE Syllabus for Class 9 Social Science 2023-24: Download PDF

CBSE Syllabus for Class 8 Maths 2024: Download PDF

NCERT Books for Class 6 Maths 2025: Download Latest PDF

CBSE Class 10 Study Timetable 2024 – Best Preparation Strategy

CBSE Class 10 Syllabus 2025 – Download PDF

CBSE Syllabus for Class 11 2025: Download PDF
Tag cloud :.
- entrance exams
- engineering
- ssc cgl 2024
- Written By Pavithra VG
- Last Modified 25-01-2023
Avogadro’s Hypothesis: Avogadro’s Law, Examples, Formula, Applications
You will find the answer to all these interesting questions in this article about Avogadro’s Hypothesis . The atomicity of water \(\left( {{{\rm{H}}_2}{\rm{O}}} \right)\) is \(3\). A molecule of water is made up of \(2\) hydrogen atoms and \(1\) oxygen atom. Now, how many hydrogen and oxygen molecules make up a molecule of water? At constant temperature and pressure, is the number of molecules in different gases like hydrogen, oxygen, steam, ammonia, etc. the same or different?
The law is named after Amedeo Avogadro, who suggested in 1812 that two identical samples of an ideal gas with the same volume, temperature, and pressure have the same number of molecules. When equal amounts of gaseous hydrogen and nitrogen are at the same temperature and pressure, they contain the same number of atoms and exhibit perfect gas behaviour.

In this article, you will explore Avogadro’s law, examples of it, Avogadro’s constant its applications in detail and more. Continue reading for more information.
What is Avogadro’s Hypothesis?
The Italian chemist Amedeo Avogadro, established a relationship between the volume of a gas and the corresponding number of molecules under a given set of conditions of temperature and pressure. This hypothesis is called Avogadro’s hypothesis. It states that, under similar conditions of temperature and pressure, an equal volume of all gases contain an equal number of molecules.
\({\rm{V}} \propto {\rm{N}}\)
Where V is the volume of the gas and N is the number of molecules.
For example, if an equal volume of four gases hydrogen \(\left( {{{\rm{H}}_2}} \right)\), oxygen \(\left( {{{\rm{O}}_2}} \right)\), chlorine \(\left( {{\rm{C}}{{\rm{l}}_2}} \right)\) and ammonia \(\left( {{\rm{N}}{{\rm{H}}_3}} \right)\) is enclosed in the different flasks of the same capacity under similar conditions of temperature and pressure, then all flasks have the same number of molecules. However, these molecules may be different in size and mass.

Avogadro’s Hypothesis and Dalton’s Atomic Theory
Avogadro’s Hypothesis is a modification of the Berzelius hypothesis. According to the Berzelius hypothesis, an equal volume of all gases under similar conditions of temperature and pressure contains an equal number of atoms.
But this hypothesis is not applicable to the chemical reactions involving gases. It was found that even fractions of atoms were involved in some chemical reactions. But in the Dalton atomic theory half of an atom of an element cannot exist. This conflict was solved by Avogadro by making a clear distinction between atom and molecule.
According to Avogadro, an atom is the smallest particle of an element that may or may not have an independent existence. In contrast, a molecule is the smallest particle of a substance (element or compound) that can exist independently.
L earn Ideal Gas Equation
Example for Avogadro’s Hypothesis: Formation of HCl Gas
One volume of hydrogen and one volume of chlorine combine to give two volumes of hydrogen chloride gas and NTP (Normal Temperature Pressure) conditions.
\({\rm{Hydrogen}} + {\rm{Chlorine}} \to {\rm{Hydrogen}}\,{\rm{chloridegas}}\)
\(1\,{\rm{Volume}}\,1\,{\rm{Volume}}\,2\,{\rm{Volumes}} = {\rm{kn}}\)
Let \(1\) volume of each gas contain n molecules.
By applying Avogadro’s hypothesis
\({\rm{Hydrogen}} + {\rm{Chlorine}} \to {\rm{Hydrogen}}\,{\rm{chloridegas}}\) \({\rm{n}}\,{\mkern 1mu} {\rm{molecules}}\,\,{\rm{n}}\,{\mkern 1mu} {\rm{molecules}}{\mkern 1mu} \,\,2{\rm{n}}{\mkern 1mu} \,{\rm{molecules}}\) \(1\,{\mkern 1mu} {\rm{molecules}}\,{\mkern 1mu} 1\,{\mkern 1mu} {\rm{molecules}}{\mkern 1mu} \,2{\mkern 1mu} \,{\rm{molecules}}\) \(\frac{1}{2}{\mkern 1mu} \,{\rm{molecules}}{\mkern 1mu} \,\frac{1}{2}{\mkern 1mu} \,{\rm{molecules}}{\mkern 1mu} \,1{\mkern 1mu} \,{\rm{molecules}}\)
This means that \(1\) molecule of hydrogen chloride contains ½ molecule of hydrogen and \(1/2\) molecule of chlorine. Now, \(1/2\) molecule of hydrogen can exist because one molecule of hydrogen contains two atoms of hydrogen, and \(1/2\) molecules of hydrogen mean one atom of hydrogen. Similarly, \(1/2\) molecule of chlorine contains an atom of chlorine because chlorine is also a diatomic molecule. Thus, one molecule of hydrogen chloride is formed from \(1\) atom of hydrogen and \(1\) atom of chlorine. This agrees with Dalton theory.
What is the Value of Avogadro Constant?
The number of molecules in one mole of gas has been determined to be \(6.022 \times {10^{23}}\). This value is known as Avogadro Constant.
According to Avagadro, all gases containing an equal amount of substances occupy the same volume at the same temperature and pressure.
\({\rm{V}} \propto {\rm{n}}\)
\({\rm{V = kn}}\)
Where \({\rm{k}}\) is the proportionality constant.
One mole, each gas at standard temperature and pressure, will have the same volume. This is known as molar volume \(\left( {{{\rm{V}}_{\rm{m}}}} \right)\). \(1\) mole of any gas at the \(273.15\;{\rm{K}}\) and \(1\) bar pressure occupies \({22.7110^{ – 3}}\;{{\rm{m}}^3}\) or \(22.71\;{\rm{L}}\).
The number of moles can be calculated by the equation,
We know that, \({\rm{n}} = \frac{{{\rm{Mass}}\,{\rm{of}}\,{\rm{gas}}}}{{{\rm{Molar}}\,{\rm{mass}}}} = \frac{{\rm{m}}}{{\rm{M}}}\)
\({\rm{V}} = {\rm{k}}\frac{{\rm{m}}}{{\rm{M}}}\)
\({\rm{M}} = {\rm{k}}\frac{{\rm{m}}}{{\rm{V}}}\)
We know that density, \({\rm{d}} = \frac{{\rm{m}}}{{\rm{v}}}\)
On rearranging,
\({\rm{M = kd}}\)
Hence, the density of a gas is directly proportional to its molar mass.

Applications of Avogadro’s Law
1. Deduction of atomicity of Elementary Gases: Atomicity of an elementary substance is defined as the number of atoms of the element present in one molecule of a substance. Example: Atomicity of oxygen \(\left( {{{\rm{O}}_{\rm{2}}}} \right)\) is \(2\) while that of ozone \(\left( {{{\rm{O}}_{\rm{3}}}} \right)\) is \(3\). Avogadro’s law helps in determining the atomicity of elementary gases such as hydrogen, oxygen, chlorine, etc. Example: Calculation of atomicity of oxygen. Consider the reaction between hydrogen and oxygen to form water vapour. Two volumes of hydrogen combined with \(1\) volume of oxygen to form two volumes of water vapour. \({\rm{Hydrogen}} + {\rm{Oxygen}} \to {\rm{Water}}\,{\rm{Vapour}}\) \(2\,{\rm{Volumes}}\,1\,{\rm{Volume}}\,2\,{\rm{Volumes}}\) Applying Avogadro’s hypothesis \({\rm{Hydrogen}} + {\rm{Oxygen}} \to {\rm{Water Vapour }}\) \(2{\rm{n}}\,{\rm{molecules}}\,{\rm{n}}\,{\rm{molecules}}\,2{\rm{n}}\,{\rm{molecules}}\) \(1\,{\rm{molecules}}\,\frac{1}{2}\,{\rm{molecules}}\,1\,{\rm{molecules}}\) Thus, \(1\) molecule of water contains \(\frac{1}{2}\) molecule of oxygen. But \(1\) molecule of water contains \(1\) atom of oxygen. Hence, \(\frac{1}{2}\) molecules of oxygen \( = 1\) atom of oxygen Or \(1\) molecule of oxygen \(= 2\) atoms of oxygen, i.e., atomicity of oxygen \(= 2\).
2. Determination of the relationship between vapour density and molar mass of a gas: The vapour density of a gas is the ratio between the mass of a certain volume of the gas to the mass of the same volume of hydrogen gas under the similar conditions of temperature and pressure. \({\rm{Vapour}}\,{\rm{density}}\,({\rm{V}}.{\rm{D}}.)\,{\rm{of}}\,{\rm{gas}} = \frac{{{\rm{Mass}}\,{\rm{of}}\,{\rm{certain}}\,{\rm{volume}}\,{\rm{of}}\,{\rm{gas}}}}{{{\rm{Mass}}\,{\rm{of}}\,{\rm{same}}\,{\rm{volume}}\,{\rm{of}}\,{\rm{hydrogen}}}}\) According to Avogadro’s hypothesis, equal volume of all gases under similar conditions of temperature and pressure contains equal number of molecules. Let the given volume of the gas and hydrogen contain n molecule at STP conditions. \({\rm{Vapour}}\,{\rm{density}} = \frac{{{\rm{Mass}}\,{\rm{of}}\,{\rm{n}}\,{\rm{molecules}}\,{\rm{of}}\,{\rm{gas}}}}{{{\rm{Mass}}\,{\rm{of}}\,{\rm{n}}\,{\rm{molecules}}\,{\rm{of}}\,{\rm{hydrogen}}}}\) \({\rm{Vapour}}\,{\rm{density}} = \frac{{{\rm{Mass}}\,{\rm{of}}\,{\rm{1}}\,{\rm{molecules}}\,{\rm{of}}\,{\rm{gas}}}}{{{\rm{Mass}}\,{\rm{of}}\,{\rm{1}}\,{\rm{molecules}}\,{\rm{of}}\,{\rm{hydrogen}}}}\) The ratio of the mass of one molecule of gas to the mass of an atom of hydrogen is called molar mass. Therefore, \({\rm{Vapour}}\,{\rm{density}} = \frac{{{\rm{Molar}}\,{\rm{Mass}}}}{{\rm{2}}}\) \({\rm{Molar}}\,{\rm{Mass}} = 2 \times {\rm{Vapour}}\,{\rm{density}}\) Vapour density is also called relative density of the gas.
3. Determination of relationship between mass and volume of the gas:
\({\rm{Molar}}\,{\rm{Mass}} = 2 \times {\rm{Vapour}}\,{\rm{density}}\)
\({\rm{Molar}}\,{\rm{Mass}} = 2 \times \frac{{{\rm{Mass}}\,{\rm{of}}\,{\rm{certain}}\,{\rm{volume}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}}}{{{\rm{Mass}}\,{\rm{of}}\,{\rm{same}}\,{\rm{volume}}\,{\rm{of}}\,{\rm{hydroen}}\,{\rm{at}}\,{\rm{STP}}}}\)
\({\rm{Molar}}\,{\rm{Mass}} = 2 \times \frac{{{\rm{Mass}}\,{\rm{of}}\,1{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}}}{{{\rm{Mass}}\,{\rm{of}}\,1{\rm{L}}\,{\rm{of}}\,{\rm{hydroen}}\,{\rm{at}}\,{\rm{STP}}}}\)
But, the mass of \({1{\rm{L }}}\) of hydrogen gas is \({\rm{0}}{\rm{.089 g}}\)
Therefore, \({\rm{Molar}}\,{\rm{Mass}} = 2 \times \frac{{{\rm{Mass}}\,{\rm{of}}\,1{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}}}{{0.089}}\)
\({\rm{Molar}}\,{\rm{Mass}} = \frac{2}{{0.089}} \times {\rm{Mass}}\,{\rm{of}}\,1{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}\)
\({\rm{Molar}}\,{\rm{Mass}} = 22.4 \times {\rm{Mass}}\,{\rm{of}}\,1{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}\)
\({\rm{Molar}}\,{\rm{Mass}} = {\rm{Mass}}\,{\rm{of}}\,22.4{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{at}}\,{\rm{STP}}\)
Thus, \(22.4{\rm{L}}\) of any gas at \({\rm{STP}}\) weighs equal to the molar mass of gas expressed in grams. This is called gram molecular volume.
You will be able to recollect Avogadro’s hypothesis, Avogadro’s law, and a comparison of Dalton’s theory after reading this article. Avogadro’s constant and applications of Avogadro’s hypothesis in identifying atomicity of elementary gases, determining a relationship between vapour density and molar mass of gas, and determining a relationship between mass and volume of the gas are all things you’re familiar with.

We have provided some frequently asked questions on Avogadro’s Hypothesis here:
Q.1. What is Avogadro’s equation? Ans: Avogadro’s equation is \({\rm{V}} = {\rm{kn}}\)
Where \({\rm{V}}\) is the volume of gas, \({\rm{n}}\) is the number of moles of gas and \({\rm{k}}\) is the proportionality constant.
Q.2. What are the applications of Avogadro’s Hypothesis? Ans: The applications of Avogadro’s Hypothesis are
- Avogadro’s law helps in determining the atomicity of elementary gases such as hydrogen, oxygen, chlorine, etc.
- It helps in the determination of the relationship between vapour density and molar mass of a gas. \({\rm{Vapour}}\,{\rm{density}} = \frac{{{\rm{Molar}}\,{\rm{mass}}}}{2}\)
- It helps in the determination of the relationship between mass and volume of the gas. \({\rm{Molar}}\,{\rm{mass}} = {\rm{Mass}}\,{\rm{of}}\,22.4{\rm{L}}\,{\rm{of}}\,{\rm{gas}}\,{\rm{STP}}\)
Thus, \({\rm{22}}{\rm{.4L}}\) of any gas at \({\rm{STP}}\) weighs equal to the molar mass of gas expressed in grams. This is called gram molecular volume.
Q.3. Why is Avogadro’s law important? Ans: Avogadro’s law is important for the calculation of the amount of gas present in a particular volume.
According to Avogadro’s law
Q.4. What is Avogadro’s hypothesis in chemistry? Ans: The Italian chemist Amedeo Avogadro established a relationship between the volume of a gas and the corresponding number of molecules under a given set of conditions of temperature and pressure. This hypothesis is called Avogadro’s hypothesis. It states that, under similar conditions of temperature and pressure, an equal volume of all gases contain an equal number of molecules.
Where \({\rm{V}}\) is the volume of the gas and \({\rm{N}}\) is the number of molecules.
Q.5. What does Avogadro’s law state? Ans: Avogadro’s law states that under similar conditions of temperature and pressure, the equal volume of all gases contains an equal number of molecules.
Q.6. What is Avogadro’s law example? Ans: An example for Avogadro’s Hypothesis is the formation of \({\rm{HCl}}\) gas. One volume of hydrogen and one volume of chlorine combine to give two volumes of hydrogen chloride gas at \({\rm{NTP}}\) (Normal Temperature Pressure) condition.
\({\rm{Hydrogen}} + {\rm{Chlorine}} \to {\rm{Hydrogen}}\,{\rm{chloride}}\,{\rm{gas}}\)
\(1\,{\rm{Volmue}}\,1\,{\rm{Volume}}\,2\,{\rm{Volumes}}\)
NCERT Solutions For Class 11 Chemistry
We hope this article on ‘Avogadro’s Hypothesis’ has helped you. If you have any queries, drop a comment below and we will get back to you.
Related Articles
1 Million Means: 1 million in numerical is represented as 10,00,000. The Indian equivalent of a million is ten lakh rupees. It is not a...
Ways To Improve Learning Outcomes: With the development of technology, students may now rely on strategies to enhance learning outcomes. No matter how knowledgeable a...
Visual Learning Style: We as humans possess the power to remember those which we have caught visually in our memory and that too for a...
NCERT Books for Class 6 Social Science 2024: Many state education boards, including the CBSE, prescribe the NCRET curriculum for classes 1 to 12. Thus,...
CBSE Syllabus for Class 9 Social Science: The Central Board of Secondary Education releases the revised CBSE Class 9 Social Science syllabus. The syllabus is...
CBSE Syllabus for Class 8 Maths 2023-24: Students in CBSE Class 8 need to be thorough with their syllabus so that they can prepare for the...
NCERT Books for Class 6 Maths 2025: The National Council of Educational Research and Training (NCERT) textbooks are the prescribed set of books for schools...
CBSE Class 10 Study Timetable: The CBSE Class 10 is the board-level exam, and the Class 10th students will appear for the board examinations for...
CBSE Class 10 Syllabus 2025: The Central Board of Secondary Education (CBSE) conducts the Class 10 exams every year. Students in the CBSE 10th Class...
CBSE Syllabus for Class 11 2025: The Central Board of Secondary Education (CBSE) has published the Class 11 syllabus for all streams on its official...
NCERT Solutions for Class 7 Science Chapter 16 Water – A Precious Resource
NCERT Solutions for Class 7 Science Chapter 16 Water – A Precious Resource: In this chapter, students will study about the importance of water. There are three...
NCERT Solutions for Class 7 Science Chapter 10 2024: Respiration in Organisms
NCERT Solutions for Class 7 Science Chapter 10 Respiration in Organisms: NCERT solutions are great study resources that help students solve all the questions associated...
Factors Affecting Respiration: Definition, Diagrams with Examples
In plants, respiration can be regarded as the reversal of the photosynthetic process. Like photosynthesis, respiration involves gas exchange with the environment. Unlike photosynthesis, respiration...
NCERT Solutions for Class 7 Science Chapter 12
NCERT Solutions for Class 7 Science Chapter 12 Reproduction in Plants: The chapter 'Reproduction' in Class 7 Science discusses the different modes of reproduction in...
NCERT Solutions for Class 7 Science Chapter 11
NCERT Solutions for Class 7 Science Chapter 11: Chapter 11 of Class 7 Science deals with Transportation in Animals and Plants. Students need to ensure...
NCERT Solutions for Class 7 Science Chapter 15: Light
NCERT Solutions for Class 7 Science Chapter 15: The NCERT Class 7 Science Chapter 15 is Light. It is one of the most basic concepts. Students...
NCERT Solutions for Class 7 Science Chapter 13
NCERT Solutions for Class 7 Science Chapter 13: Chapter 13 in class 7 Science is Motion and Time. The chapter concepts have a profound impact...
NCERT Solutions for Class 7 Science Chapter 14: Electric Current and its Effects
NCERT Solutions for Class 7 Science Chapter 14: One of the most important chapters in CBSE Class 7 is Electric Current and its Effects. Using...
General Terms Related to Spherical Mirrors
General terms related to spherical mirrors: A mirror with the shape of a portion cut out of a spherical surface or substance is known as a...
Animal Cell: Definition, Diagram, Types of Animal Cells
Animal Cell: An animal cell is a eukaryotic cell with membrane-bound cell organelles without a cell wall. We all know that the cell is the fundamental...
NCERT Solutions for Class 10 Science 2024 – Download PDF
NCERT Solutions for Class 10 Science: The National Council of Educational Research and Training (NCERT) publishes NCERT Solutions for Class 10 Science as a comprehensive...
NCERT Books for Class 12 Chemistry 2024: Download PDF
NCERT Books for class 12 Chemistry: NCERT publishes chemistry class 12 books every year. The NCERT chemistry class 12 books are essential study material for...
CBSE Class 9 Mock Tests 2025: Attempt Online Mock Test Series (Subject-wise)
We all have heard at least once that the secret to success is practice. Some of you could say it's a cliché, but those who...
NCERT Books for Class 10 Maths 2025: Download Latest PDF
NCERT Books for Class 10 Maths: The NCERT Class 10 Maths Book is a comprehensive study resource for students preparing for their Class 10 board exams....
Arc of a Circle: Definition, Properties, and Examples
Arc of a circle: A circle is the set of all points in the plane that are a fixed distance called the radius from a fixed point...
CBSE Class 10 Mock Test 2025: Practice Latest Test Series
CBSE Class 10 Mock Test 2025: Students' stress is real due to the mounting pressure of scoring good marks and getting into a renowned college....
NCERT Solutions for Class 10 2024: Science and Maths
NCERT Solutions for Class 10 2024: Students appearing for the CBSE Class 10 board exam must go through NCERT Solutions to prepare for the exams...

39 Insightful Publications

Embibe Is A Global Innovator

Innovator Of The Year Education Forever

Interpretable And Explainable AI

Revolutionizing Education Forever

Best AI Platform For Education

Enabling Teachers Everywhere

Decoding Performance

Leading AI Powered Learning Solution Provider

Auto Generation Of Tests

Disrupting Education In India

Problem Sequencing Using DKT

Help Students Ace India's Toughest Exams

Best Education AI Platform

Unlocking AI Through Saas

Fixing Student’s Behaviour With Data Analytics

Leveraging Intelligence To Deliver Results

Brave New World Of Applied AI

You Can Score Higher

Harnessing AI In Education

Personalized Ed-tech With AI

Exciting AI Platform, Personalizing Education

Disruptor Award For Maximum Business Impact

Top 20 AI Influencers In India

Proud Owner Of 9 Patents

Innovation in AR/VR/MR

Best Animated Frames Award 2024
Trending Searches
Previous year question papers, sample papers.
Practice Avogadros Hypothesis Questions with Hints & Solutions

Practice Avogadros Hypothesis Questions with Solutions & Ace Exam
Enter mobile number.
By signing up, you agree to our Privacy Policy and Terms & Conditions

IMAGES
VIDEO
COMMENTS
Avogadro's law, also known as Avogadro's principle or Avogadro's hypothesis, is a gas law which states that the total number of atoms/molecules of a gas (i.e. the amount of gaseous substance) is directly proportional to the volume occupied by the gas at constant temperature and pressure.
Avogadro's law (sometimes referred to as Avogadro's hypothesis or Avogadro's principle) or Avogadro-Ampère's hypothesis is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific case of the ideal gas law.A modern statement is: Avogadro's law states that "equal volumes of all gases, at the same temperature and pressure, have the ...
Avogadro's law is also known as Avogadro's hypothesis or Avogadro's principle. It is related to the other ideal gas laws: Boyle's law (1662), Charles's law (1787) and Gay-Lussac's law (1808). French physicist and mathematician André-Marie Ampère published the same law as Avogadro, but in 1814. ... Modern Chemistry. Holt, Rinehart ...
Avogadro's number. Avogadro's law, a statement that under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of molecules. This empirical relation can be derived from the kinetic theory of gases under the assumption of a perfect (ideal) gas. The law is approximately valid for real gases ...
Avogadro's Law Formula. Avogadro's law's mathematical formula can be written as: V ∝ n or V/n = k. Where "V" is the volume of the gas, "n" is the amount of the gas (number of moles of the gas) and "k" is a constant for a given pressure and temperature. Avogadro's law formula describes how equal volumes of all gases contain ...
Italian physicist Amedeo Avogadro was the first to state the hypothetical law in 1811. Avogadro's Law Formula. According to Avogadro's hypothesis, the volume (V) of a gas is directly proportional to the number of moles (n) [1-4].
Explore Avogadro's Law with this engaging lesson. Discover how the Italian chemist Amedeo Avogadro's experiments with tiny particles led to the postulation that equal volumes of gas at the same temperature and pressure contain equal number of particles. This fundamental principle forms the basis of the ideal gas equation, a cornerstone in the ...
Definition and Example. Italian chemist Amedeo Avogadro proposed Avogadro's Law in 1811 to describe the behavior of gases at equal pressure. DEA/CHOMON/Getty Images. Avogadro's Law is the relation which states that at the same temperature and pressure, equal volumes of all gases contain the same number of molecules.
Contrary to the beliefs of generations of chemistry students, Avogadro's number—the number of particles in a unit known as a mole—was not discovered by Amadeo Avogadro (1776-1856).
The mole and Avogadro's number. One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or Avogadro's constant. The concept of the mole can be used to convert between mass and number of particles..
Avogadro's early days. Lorenzo Romano Amedeo Carlo Avogadro, the Count of Quaregna and Cerreto, was born in the Piedmontese city of Turin in the kingdom of Sardinia on 9 August 1776. He was the son of a prominent civil servant who was charged under the Napoleonic rule of 1799 to reorganise the Piedmont Government.
Many of Avogadro's pioneering ideas and methods anticipated later developments in physical chemistry. His hypothesis is now regarded as a law , and the value known as Avogadro's number (6.02214076 × 10 23 ), the number of molecules in a gram molecule, or mole , of any substance, has become a fundamental constant of physical science .
In 1811, Amedeo Avogadro explained that the volumes of all gases can be easily determined. Avogadro's hypothesis states that equal volumes of all gases at the same temperature and pressure contain equal numbers of particles. Since the total volume that a gas occupies is made up primarily of the empty space between the particles, the actual size of the particles themselves is nearly negligible.
Avogadro's law states that the volume of a gas is directly proportional to the number of moles of gas when the temperature and pressure are held constant. The mathematical expression of Avogadro's law is. V = k × n and V 1 n 1 = V 2 n 2. where n is the number of moles of gas and k is a constant. Avogadro's law is in evidence whenever you ...
A great example of Avogadro's law is the way that we breathe. When a person breathes in, the molar amount of air in their lungs goes up, and so does the volume of their lungs (expansion of the lungs). The way car tires lose air is another common example of Avogadro's law. When the air that was trapped in the tire gets out, the amount of air ...
Avogadro's law is a gas law and is also referred to as Avogadro's hypothesis or Avogadro's principle. It states that the total number of molecules or atoms of a gas is directly proportional to the volume that the gas occupies at a constant temperature and pressure. This law is much related to the ideal equation of the gas because it links ...
Avogadro's Hypothesis, introduced by Amaedo Avogardro in 1811, explains that in every gas the number of molecules is proportional to the volume. He replaced the term 'atoms' in the Berzelius hypothesis with the term 'molecules' and postulated a law, popularly known as Avogadro's hypothesis. Avogadro's hypothesis states, "Equal ...
What is Avogadro's hypothesis in chemistry? Ans: The Italian chemist Amedeo Avogadro established a relationship between the volume of a gas and the corresponding number of molecules under a given set of conditions of temperature and pressure. This hypothesis is called Avogadro's hypothesis. It states that, under similar conditions of ...
Mercury could have a thick underground layer of diamonds, scientists say. Some of the stones may have found their way to the surface, according to a recent study.