- Market Access US & International
- Market Access Europe
- MDR / IVDR Consulting
- IVD Admission Strategy
- AI Medical Devices
- And more…
- Quality management systems (ISO 13485)
- ISO 13485 audits
- Quality management representative
- Quality management system as a service
- Software (IEC 62304, FDA)
- Risk Management (ISO 14971)
- Clinical Evaluation
- Performance evaluation of IVDs
- Electrical Safety & IEC 60601
- Human Factors / Usability (IEC 62366 and FDA)
- FDA relevant documents
- Human Factors Research
- Safety and EMC test laboratory
- Biological safety
- Computer System Validation
- Seminar Overview
- Digital Transformation
- Medical Device University
- Post Market Tools
- Our Mission
- Certificates
- Our Customers
- Current Vacancies
- Why the Johner Institute?
- Regulatory Affairs
- Laboratory products for “Research Use…

Laboratory products for “Research Use Only” (RUO) – often a dangerous claim
Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs). Nevertheless, some medical laboratories still use RUO products in diagnostic procedures, sometimes even with the knowledge of the manufacturers. This can have consequences – not just for manufacturers and operators but for patients as well.
In this article, you will learn
- what the “Research Use Only” (RUO) label means,
- what the requirements for RUO products are,
- how to avoid legal problems, and
- what alternatives there are to RUO products.
1. “Research Use Only” – what does it mean?
Products labeled “For Research Use Only” are hardly subject to any regulatory controls. Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) aims to distance itself from RUO products clearly:
The scope of application of this Regulation should be clearly delimited from other legislation concerning products, such as medical devices, general laboratory products and products for research use only. IVDR Foreword (7)
a) Institutions affected
The following institutions, in particular, use RUO products:
- Medical laboratories can utilize RUO products, but doing so designates them as the manufacturer, carrying all the associated consequences.
- If medical laboratories utilize RUO products for purposes beyond research, this can potentially render them liable for damages and subject to criminal liability in the worst-case scenario.
You can find more information on “Lab Developed Tests” in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Manufacturers can incorporate RUO products as components in their IVD, but they are subsequently responsible for ensuring the conformity of the end device with the IVDR. The RUO labeling of the component is not mandatory.
- If manufacturers designate their devices as “RUO,” the intended use of these devices must be interpreted accordingly and, if required, substantiated. For instance, reasonably foreseeable misuse should be taken into account. The RUO label should not be applied to the device as a mere “protective claim,” as this may result in legal consequences.
b) Definition
There is no standardized definition for “Research Use Only” (RUO) products. Generally, they can be understood as products designed for analysis intended solely for scientific research purposes, as the name implies. Their main distinction from medical devices lies in their inability to be used for medical purposes.
Nevertheless, the interpretation of “Research Use Only” varies between Europe and the USA.
Definition in Europe
In Europe, the MEDDEV 2.14/2 guidance document ( IVD Guidance: Research Use Only products – A guide for manufacturers and notified bodies ) provides a definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can still be considered the state of the art.
MEDDEV 2.14/2 states:
“for a product to be categorized as an RUO product it must have no intended medical purpose or objective.” Source: MEDDEV 2.14/2 rev.1
This means that an RUO product must not have a medical purpose, even not a rudimentary one.
This also applies to tests developed in-house (Laboratory Developed Tests) that are only used in a health institution for research purposes.
The IVDR also addresses RUO products. Article 1 (3) a) of the IVDR excludes RUO products from its scope:
This Regulation does not apply to: (a) products for general laboratory use or research-use only products, unless such products, in view of their characteristics, are specifically intended by their manufacturer to be used for in vitro diagnostic examination; Source: IVDR, Article 1 (3) a)
Furthermore, Article 2 (45) specifies:
“A device intended to be used for research purposes, without any medical objective , shall not be deemed to be a device for performance study;” IVDR, Article 2 (45)
Devices for performance studies are:
“‘device for performance study ’ means a device intended by the manufacturer to be used in a performance study” IVDR, Article 2 (45)
The IVDR thus distinguishes RUO products from IVDs and products for performance studies. The EU regulation also highlights the lack of a medical intended purpose for RUO products.
Definition in the USA
In 2013, the FDA published a guidance document on RUOs entitled “ Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only .”
This guidance defines RUO products as follows:
“ An RUO product is an IVD product that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812” [NB: Part 812 concerns the provision of devices for performance evaluation purposes as a preliminary step to IVDs] FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
Some examples of products that the FDA believes fall into this research phase of development are:
- Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.
However, the FDA further specifies:
“FDA also recognizes that there are certain products, such as instruments, systems, and reagents that are labeled for research use only and intended for use in the conduct of nonclinical laboratory research with goals other than the development of a commercial IVD product […].” FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”, Chapter III A
And subsequently gives examples of such research purposes in which the product itself is not the subject of research.
The FDA thus sees two “types” of RUO products: First, IVD devices whose development is ongoing and which are themselves the subject of the research purpose, and second, products for nonclinical research.
In both cases, the FDA requires a clearly visible RUO label to be affixed to the products. The RUO label is intended to prevent use for clinical diagnostics, patient management, and other investigations with a medical purpose.
c) What are the consequences of using the “Research Use Only” label?
Normally, IVDs are subject to regulatory requirements (for example, according to the IVDR or FDA) based on their risk class.
However, RUO products do not fall within the definition of “in vitro diagnostic medical devices” given by the IVDR or the relevant FDA regulations . This means that these regulations do not apply to RUO products.
‘ In vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state; (b) concerning congenital physical or mental impairments; (c) concerning the predisposition to a medical condition or a disease; (d) to determine the safety and compatibility with potential recipients; (e) to predict treatment response or reactions; (f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices.
Source: Article 2 IVDR
“In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
Source: 21 CFR 809.3
However, RUO products do not automatically fall entirely outside the regulatory scope in the EU. Depending on the product, they may still have to comply with requirements that are not specifically intended for IVDs (such as the REACH regulation for chemicals or the Machinery Directive ).
Read more about the Machinery Directive: Which parts apply to medical devices .
Since RUO products are subject to considerably fewer controls than IVDs, it is necessary to severely restrict their use. Therefore, in particular they may not be used to
- make diagnoses and
- conduct performance studies.
2. Use and misuse of “Research Use Only” labels
A) what should ruo products be used for.
As the name “For Research Use Only” indicates, products with RUO labeling are intended for research purposes only. RUO products are particularly attractive for the research sector due to the simplified process and lower hurdles for placing them on the market.
MEDDEV. 2.14/2 rev.1 provides a precise list of areas where RUO products may potentially be used:
- basic research
- pharmaceutical research
- better identification and quantification of individual chemical substances or ligands in biological specimens
- in-house manufacturing of so called “Laboratory Developed Tests” for research purposes
And of areas where the use of RUOs is expressly not permitted:
- use of raw materials which are labeled “For Research Use Only” but which are incorporated into a finished product
- so called “research use products” being tested against a comparator IVD product that bears the CE mark
- products for market studies/feasibility studies
These products can be assigned a medical purpose.
b) What RUO products are often used for
However, the low hurdles are also the reason why RUO products are often used for purposes they are not intended for. This poses significant dangers for manufacturers, operators, and patients.
Sale of RUO products to medical laboratories
RUO products are sold by manufacturers to medical laboratories. Although doctors sometimes also conduct research, this is not really the main purpose of a medical laboratory.
Therefore, when discussing sales with doctors, it should always be assumed that there is a medical reason behind the use of the product. This means that anyone who knowingly sells RUO products to medical laboratories is potentially under suspicion of using the pretext “For Research Use Only” to ignore an intended medical purpose and thus avoid responsibility for a medical device.
Avoid reference to any specific diagnostic procedures in your advertising materials for products that clearly do not have a medical purpose. You should always stay on the technical or purely analytical level.
Use of RUO products in medical laboratories
The issue of selling RUOs to medical laboratories is not limited to manufacturers alone. The laboratories themselves may also not be acting in line with their status as operators and may, as a result, be liable under certain circumstances.
- Medical laboratories are free to develop in-house tests themselves. In such cases, RUO products are often used in diagnostic procedures. The laboratory bears full responsibility for these tests. Even under the IVDD, MEDDEV 2.14/2 saw this topic critical. However, with the IVDR, the EU is explicitly placing more restrictions on the routine use of such Lab Developed Tests.
Read more in our article The EU regulates medical laboratories – Are Laboratory Developed Tests still allowed?
- Due to the low regulatory hurdles, purchasing RUO products is very affordable. As a result, medical laboratories prefer them over expensive CE-IVD devices if they can achieve the same level of performance. Nevertheless, the use of RUO products for purposes other than research, even in cases where they provide similar results, is not permitted.

3. Consequences of incorrect classification
Lack of controls can have a negative effect on quality. As a result, the relevant authorities (e.g., authorities during inspections) take a closer look at whether a product is actually intended “For Research Use Only.”
Manufacturers should also be aware that simply sticking an RUO label on a product does not on its own mean that the product no longer has to comply with requirements for IVDs that would otherwise apply.
The RUO status is determined solely by the actual intended use of a device. To this end, authorities (both European and FDA) also use marketing material or other information as evidence.
Manufacturers and operators who misuse the RUO label could face severe penalties, as such behavior can cause serious harm to patients or even the general public.
a) Consequences for manufacturers and operators
Improperly selling IVDs with an RUO label or using RUO products for purposes other than research is not a trivial offense. Manufacturers who intentionally conceal or attempt to conceal a diagnostic purpose behind the RUO label should anticipate legal consequences in Germany. The same applies for operators who misuse RUO products. There is the possibility of a fine or even prison sentences. In addition, there is potential liability for harm suffered by patients.
b) Consequences in the USA
There are also severe penalties in the USA. If an RUO label is deemed to have been incorrectly used for a product, the product would be considered misbranded under sections 502(a) and 502(o) of 21 US Code, 352(a), 352(o) [A1] and would be considered adulterated under section 501(f) of 21 US Code 351(f).
c) Consequences for patients
However, the consequences can be even worse for patients. After all, the regulatory requirements for IVDs aren’t just plucked out of thin air to annoy manufacturers and operators. The regulations are intended to protect patients against incorrect results and subsequent wrong decisions. False-negative results can lull patients into a false sense of security and an existing undetected disease may worsen. One example would be the metastasis of an undetected cancer due to a test not performing as intended.
Some incorrect diagnoses could even be so severe that they can cause the death of a lot of people: an undetected viral infection can cost many lives in the early stages of an epidemic or pandemic, as the coronavirus pandemic sadly demonstrated.
4. Alternatives to “Research Use Only” products
To avoid legal problems and risks to third parties, manufacturers and users should use general laboratory equipment as an alternative to RUO products.
There are laboratory products that obviously have no specific medical purpose, such as
- pure chemicals,
- culture media,
- reaction vessels,
- washing solutions,
- qPCR cycler,
- sequencers,
- centrifuges.
Read more on the topic here: General laboratory equipment: What manufacturers and laboratories need to know to avoid problems and unnecessary expense
5. Ways to protect yourself
Manufacturers, operators, and patients can take the following steps to avoid legal and other negative consequences when using RUO products:
a) Manufacturers
In the case of manufacturers, it is particularly important that they narrowly define the intended purpose of their product.
Analyte specific reagents should only be labeled as RUO products for specific non-medical purposes.
SARS-CoV-2 and its mutations: a test kit that uses specific primers and probes to distinguish the variants B.1.1.7 (alpha variant) and B.1.351 (beta variant) from the initial variant following a positive result may be an RUO product if it is only intended to be used to determine the prevalence of the variant in the population.
A specific intended purpose in this case would be: “ Intended solely for epidemiological research for the purpose of surveying the prevalence of SARS-CoV-2 variants in the general population. ”
If a medical laboratory subsequently, based on new findings, used this test to provide the best possible treatment for infection by a specific variant, this would be an off-label use. The laboratory would then be responsible for the test’s conformity.
Tip: Provided the manufacturer did not advertise the product with this clinical benefit, it would be adequately protected.
b) Operators
Operators should record exactly for what they use IVDs and RUO products.
Medical laboratories are operators of medical devices and IVDs and, therefore, are responsible for only using medical devices according to their intended purpose and in accordance with the generally accepted rules of the technology. This is stipulated in Section 4 of the German Medizinprodukte-Betreiberverordnung (MPBetreibV).
To be on the safe side, laboratories should keep a record of which medical devices and IVDs are in operation and routine use. This record should include a reference to the applicable test procedure and the intended purpose of the IVD.
This record can also be used to identify investigational procedures for which there are no adequate CE-IVDs available on the market. The lack of alternatives would justify the use of RUOs in validated processes as in-house IVD , provided that the laboratory verifies and demonstrates that the general safety and performance requirements and the additional requirements of Article 5(5) of the IVDR are met.
Read more about the requirements for LDTs in our article .
c) Patients
Patients lack the knowledge to recognize what is and isn’t an RUO on their own. They are often given little to no information about the test they are undergoing. So, patients should follow this basic rule: ask your doctor or pharmacist!
- Patients can ask for the complete test report from the laboratory so that they can get a second opinion in case of doubt. The report should also indicate which specific test was performed.
- Patients should inform themselves about how “well” or “poorly” a test works, as well as the benefit-risk ratio.
- In the future, patients and doctors will also be able to get information about medical devices from EUDAMED and use this information to decide whether or not the test was performed with certified and thus legally compliant IVDs.
6. Conclusion
In the opinion of the EU Commission and the FDA, products “For Research Use Only” have no place in diagnostics. To be used for diagnostic purposes, products have to go through the necessary controls. But these controls do not apply to RUO products.
Anyone who ignores this prohibition and uses or sells RUO products for purposes other than pure research is playing with fire. Manufacturers and operators run the risk of legal trouble and could even endanger patients’ health. Therefore, RUO products should only be used for research purposes. For other uses, manufacturers and operators should use the alternatives mentioned.
If you, as a manufacturer or medical laboratory, find that an RUO product is particularly well-suited for in vitro diagnostics, consider whether further development and conformity assessment to make it an IVD is worthwhile.
Thanks to Dr. Boris Handorn , lawyer and partner at PRODUKTKANZLEI , Augsburg, for his valuable input on this article.
Benefit from the support of our IVD experts:
- They will help you qualify your devices or examination procedures, for example, with in-house workshops on approval strategy and in-house IVDs.
- They provide you with expert opinions on the qualification of your device, which you can submit to your customers and/or notified bodies.
- They support you in all activities up to the “certification” of your device (e.g., performance evaluation) and beyond (e.g., post-market surveillance).
Or use our e-learning platform : Learn how to meet the regulatory requirements and get access to our IVD-specific templates and tutorials on how to get your device approved.
Change history
- 2024-02-01 Complete revision; section “The thing with analyte-specific reagents” removed; shortening of chapter 4 (deletion of subchapters a) to c)); reference to article on general laboratory equipment
- 2021-11-16 First publication
More Articles
Medical Device Regulation MDR (2017/745) Status 2024 August 27, 2024
Electronic instructions for use for medical devices (EU law) August 22, 2024
I cannot access the specific URL you provided. However, if it’s about “For Research Use Only (RUO)” in regulatory affairs, a comment could emphasize the critical role of clear labeling and compliance in ensuring safety and integrity in research settings, promoting transparency and trust in scientific practices.
Hi RRMA Global,
Thank you for the comment! There seems to have been a mismatch in the links. These should now all be correct.
Kind regards Tea Bodrusic
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
Notify me when new comments are added.
We need your consent before you can continue on our website. If you are under 16 and wish to give consent to optional services, you must ask your legal guardians for permission. We use cookies and other technologies on our website. Some of them are essential, while others help us to improve this website and your experience. Personal data may be processed (e.g. IP addresses), for example for personalized ads and content or ad and content measurement. You can find more information about the use of your data in our privacy policy . You can revoke or adjust your selection at any time under Settings .
- External Media
Individual Privacy Preferences
Cookie Details Privacy Policy Imprint
If you are under 16 and wish to give consent to optional services, you must ask your legal guardians for permission. We use cookies and other technologies on our website. Some of them are essential, while others help us to improve this website and your experience. Personal data may be processed (e.g. IP addresses), for example for personalized ads and content or ad and content measurement. You can find more information about the use of your data in our privacy policy . Here you will find an overview of all cookies used. You can give your consent to whole categories or display further information and select certain cookies.
Accept all Save
Essential cookies enable basic functions and are necessary for the proper function of the website.
Show Cookie Information Hide Cookie Information
| Name | |
|---|---|
| Provider | Owner of this website, |
| Purpose | Saves the visitors preferences selected in the Cookie Box of Borlabs Cookie. |
| Cookie Name | borlabs-cookie |
| Cookie Expiry | 1 Year |
Content from video platforms and social media platforms is blocked by default. If External Media cookies are accepted, access to those contents no longer requires manual consent.
| Accept | |
|---|---|
| Name | |
| Provider | Google Ireland Limited, Gordon House, Barrow Street, Dublin 4, Ireland |
| Purpose | Used to unblock YouTube content. |
| Privacy Policy | |
| Host(s) | google.com |
| Cookie Name | NID |
| Cookie Expiry | 6 Month |
Privacy Policy Imprint

In Vitro Diagnostic Use (IVD) versus Research Use Only (RUO) in the Clinical Laboratory
by Lindsey Drake | Clinical , Molecular

Publish Date: April 3, 2023
Previously, in this series we highlighted the importance of external and third-party quality control products in the clinical laboratory, emphasizing that quality control, by design, should add layers of removal from the assay to ensure the utmost degrees of objectivity. External, third-party quality control materials fulfill this QC requirement by removing bias in the quality control process. However, in addition to adding objectivity to the quality control process, it is critical that clinical laboratories consider the quality and the regulatory status of their quality control products.
The IVD and RUO labels are so commonplace in diagnostic laboratories that they easily go unnoticed. Clinical laboratory professionals may not pause to remember that these labels stand for In Vitro Diagnostics (IVD) and Research Use Only (RUO). Even clinical laboratory professionals who are familiar with these regulatory designations for assays or instruments sometimes do not realize the full significance that these labels have for certain products, including quality controls. Therefore, even though the quality and the regulatory status of quality control products is essential to consider, many clinical laboratories may not know the status of their quality control materials and products. This lack of understanding inadvertently puts both patients and the clinical laboratory at risk.
This blog post will examine the differences between RUO and IVD and the importance of choosing the right quality control products in the clinical laboratory. For additional information on RUO and IVD, check out our webinar highlighting the critical differences between RUO and IVD and the importance of choosing the right clinical diagnostic products. We partnered with CAP Today and industry expert Dr. Sebastian Grömminger to bring you this webinar.
Research Use Only (RUO)
RUO stands for Research Use Only. The RUO label serves as a warning to clinical laboratory professionals that the materials in question are not intended for use with patient diagnostics. RUO products are in the laboratory phase of development and must have no intended medical purpose or objective, as these materials do not require validations or regulatory compliance. 2 RUO materials are not defined in the EU’s In Vitro Diagnostic Medical Devices Regulation (IVDR) and do not have any regulatory requirements. Therefore, RUO materials are to be used only for testing with no direct impact on patient diagnostics.
According to United States Food and Drug Administration (FDA), 3
- Similar to the case in the EU, RUO refers to products in the “laboratory phase of development,” which are “not approved for clinical diagnostic use”
- RUO products are “exempt from most regulatory controls,” so it is therefore “important that they are not distributed for clinical diagnostic uses”
- All product labeling for these products must bear a prominent user notification: “‘For Research Use Only. Not for use in diagnostic procedures.’”
- RUO Labeling is intended to “serve as a warning, to prevent such products” from being used in manners that will impact patient testing and treatment outcomes.
- Companies selling RUO materials are limited in their marketing in some regions. In these regions, manufacturers may be forbidden from providing technical support for RUO materials. Availability of technical support is thus an important advantage of IVD products. Since the clinical diagnostic field is complex, having experts to rely on is an indispensable service exclusive to IVD controls.
Since RUO materials are in the laboratory phase of development, their inappropriate use in clinical diagnostics may pose unnecessary threats to diagnostic precision, laboratory efficiency, operating margins, and risk management systems. Therefore, clinical laboratories should not use these materials for reporting patient results. Thus, the RUO label is a warning to the clinical laboratory professional that this material is not intended for use in clinical diagnostics. Rather than using the RUO material, a clinical laboratory professional should look for the right IVD product for any test that could directly impact patient health outcomes.
In Vitro Diagnostic (IVD)
IVD stands for In Vitro Diagnostic. In contrast to RUO, according to global regulations and standards, IVD products are used for medical applications or purposes. According to the FDA, “In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.” 4 According to the IVDR in the European Union and the UK Medicines and Healthcare Products Regulatory Agency (MHRA), IVD medical devices have a medical application or purpose. 4
IVD products must undergo extensive validations to be registered by the FDA, IVDR, and the MHRA. Registrations are required in most countries. Table 1 highlights the regulatory criteria comparison between IVD and RUO products. As shown in the table, IVD products are subject to numerous regulatory requirements, from labeling to post-market surveillance. 6,7 RUO materials do not share these requirements, thus laboratories using RUO materials take on increased risk. 8
Since the manufacturer of the IVD product has invested in extensive studies to validate the performance of IVD product, the clinical laboratory can benefit by reducing the validations required on part of the clinical laboratory. Laboratories choosing to use RUO materials will need to perform more extensive validations than those using IVD products. Thus, clinical laboratories choosing RUO materials take on increased risk and must invest more time into validation and documentation efforts.
| Specific standard | No | ISO13485 |
| Labeled for a specific clinical or diagnostic use | No | Yes |
| Can be used for specific clinical diagnosis | No | Yes |
| Subject to QS Regs 21CFR820 | No | Yes |
| Registration and listing required | No | Yes |
| Adverse event reporting required | No | Yes |
| Post market surveillance | No | Yes |
| Premarket notification requirements | No | Yes (aligned with class) |
Table 1. IVD vs. RUO Regulatory Criteria Comparison. As shown in the table, IVD products are subject to numerous regulatory requirements from labeling to post market surveillance. RUO materials do not share these requirements, thus laboratories using RUO materials take on increased risk.
IVD products also have stringent product development requirements according to IVDR and FDA as shown in Table 2. IVD products have requirements for clinical performance, analytical performance, manufacturing and reproducibility, shipping and stability, failure mode analysis and labeling requirements. RUO materials do not specify requirements for these processes. Again, IVD products are required to demonstrate stringent performance characteristics and regulatory requirements whereas RUO materials do not.
| Clinical performance | |
| Analytical performance | |
| Manufacturability and reproducibility | |
| Shipping and stability | |
| Failure mode analysis | |
| Label requirements |
Table 2. IVD: Product Development Requirements. Here we see the product development process requirements under IVDR and FDA for IVD Designation. The IVDR is aligned with FDA here. As shown in the table, IVD products have requirements for clinical performance, analytical performance, manufacturing and reproducibility, shipping and stability, failure mode analysis and labeling requirements. RUO materials do not specify requirements for these processes.
As mentioned above, the unavailability of technical support for RUO materials in certain regions may further limit their ability to fulfill the demands of quality control in clinical settings. Since quality control aims to provide confidence in the laboratory’s analytical processes, QC must bring together the right technical expertise and the right products. Given the complexity of clinical diagnostics, technical support is an indispensable service offered by IVD manufacturers.
Sometimes, IVD products may not be available for a particular assay. In certain cases, non-IVD-labelled products may be permissible in clinical diagnostics, such as when testing materials receive Emergency Use Authorizations because IVD products are not yet available. When used in these extenuating circumstances, non-IVD-labelled products will require the laboratory professional to perform additional steps to satisfy regulatory demands for quality control.
Quality controls help reveal blind spots, biases, material defects, and other causes of inaccurate diagnostic results. By design, quality control allows the clinical laboratory to test their analytical processes in a controlled manner to ensure assays and instruments perform as expected before their use for patient samples. Because approximately 70% of healthcare decisions rely on the results of laboratory testing, accurate diagnostic processes are critical to patient care. Therefore, quality control is foundational to the quality management system of a clinical laboratory, and its role cannot be understated.
Because of this critical role, clinical laboratories must not only use quality control materials with an intended use in clinical diagnostics, but also must choose products meeting the highest standards for quality, precision, and scientific rigor. When clinical laboratories choose an IVD product, they put their confidence not only in the regulatory status of the material for use in clinical diagnostics, but also confidence that manufacturer has invested time and care to ensure the product has been properly validated and subject to stringent quality requirements before its release as a product offering.
Clinical laboratories should keep in mind that, according to global regulations (e.g., European Commission, FDA, etc.), RUO materials have no place in routine clinical diagnostics. Clinical laboratories that choose to use RUO materials are putting their patients and laboratory at risk. In addition to not having an intended use in clinical diagnostics, RUO materials are exempt from most regulatory controls which may impact product quality. Additionally, a lack of technical support may intensify these drawbacks.
Choosing IVD quality control products is essential in the clinical laboratory as these products have undergone extensive validations required to be registered with FDA, European Commission, MHRA, and other regulatory bodies for an intended use in clinical diagnostics. An IVD label signifies exactly what you look for in an external, third-party control: uncompromising quality, according to the highest standards. The IVD label represents product quality and confidence and strengthens the clinical laboratory’s risk management program. The IVD label allows technical support assistance. Reliance on a reputable third-party, IVD quality control manufacturer gives back the clinical laboratory time, money, and peace of mind.
Find IVD controls for your assays at Microbiologics.com .
1. European Commission. (2004, February). Guidance document – In vitro diagnostic medical devices – Research Use Only products – MEDDEV 2.14/2 rev.1. European Commission. https://ec.europa.eu/docsroom/documents/10292/attachments/1/translations
2. Center for Devices and Radiological Health. (2013, November 25). Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only. Guidance for Industry and Food and Drug Administration Staff. U.S. Food and Drug Administration. https://www.fda.gov/media/87374/download
3. Center for Devices and Radiological Health. (2013, November 25). Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only. Guidance for Industry and Food and Drug Administration Staff. U.S. Food and Drug Administration. https://www.fda.gov/media/87374/download
4. Center for Devices and Radiological Health. Overview of IVD Regulation. U.S. Food and Drug Administration. Retrieved March 6, 2023, from https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation
5. Regulation (EU) 2017/746 In Vitro Diagnostic Medical Devices (IVDR) https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0746&from=EN
6. Center for Devices and Radiological Health. (2021, October 21). Overview of IVD Regulation. U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation#1
7. 21CFR809, Subpart B, In Vitro Diagnostic Products for Human Use. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=809&showFR=1
Written by Lindsey Drake
You may also like.


MLS and MLT Career Opportunities
As a manufacturer of quality controls used in clinical diagnostics, we see firsthand the difficulties that laboratory...

The Challenges of Diagnosing and Treating Secondary Infections
In the past two decades, there have been six major global outbreaks of infectious diseases. While these infections may...

- Pharmaceutical
- Events and Webinars
- Uncategorized

1-800-599-2847 microbiologics.com [email protected]
QUICK LINKS
CATEGORIES RESOURCES ABOUT US CONTACT US SITE MAP PRIVACY POLICY
An Introduction to Research Use Only (RUO)
In this blog, we recap our eBook, “An Introduction to Research Use Only (RUO)” – Click HERE to download the entire publication.
Learn how it differs from adjacent labels, the FDA and EU guidance, its appropriate use, and the consequences of mislabeling products RUO.
Introduction
In the complex world of medical device development, regulation, and distribution, finding the appropriate label to put on a device may not be simple. When is one label appropriate over another? Does a device need to go through additional testing, verification, or validation? And what are the consequences of using the wrong label? In this eBook, we’ll cover the differences between Research Use Only (RUO) and a medical device – although, it’s generally a very clear distinction.
Using the right language and label is critical to complying with best practices. This is why Regulatory Affairs works with the regulatory bodies to ensure that the limitations of the product are properly documented. In a rush to get products to market, it may be tempting to use a Research Use Only (RUO) label to avoid additional regulatory processes while still empowering other researchers and developers. However, there are risks to using the RUO label inappropriately that can have serious consequences for developers, users, and patients. In fact, mislabeling a product is illegal, and punishable. You can see an example warning letter the FDA sent to Carolina Liquid Chemistries Corp after finding intentional mislabeling in 2019 here.
This introduction will provide an overview of the Research Use Only label, how it differs from similar, adjacent labels, its appropriate use, and the consequences of mislabeling products RUO.
What is Research Use Only (RUO)?
The label Research Use Only (RUO) is generally used to indicate products that are intended for scientific research only. They cannot be used for diagnostic or medical purposes. However, there is no standard definition of “research use only,” and the label has slightly different meanings in the European Union and the United States. With the IVDR regulations, RUO products that are being used in the LDT space are going to be revisited and potentially reclassified as a medical device. With this new classification, teams will likely need to follow design controls, best practices, and industry standards.
What is the FDA guidance on Research Use Only products?
Under the FDA’s guidance issued in 2013 , a product labeled Research Use Only is an In Vitro Diagnostic (IVD) product “that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812.” The agency includes in this category:
- “Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- “Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- “Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.”
The European guidance document MEDDEV 2.14/2 states that a product categorized as an RUO product “must have no intended medical purpose or objective.” The guidance does exempt some tests developed for in-house use as long as the products are not sold to other companies. Some examples of items that can be classified as “research use only” under this exemption include PCR enzymes, gel component agars, and primers.
RELATED: FDA released new draft guidance of premarket submissions for medical devices – are you ready?
What is the difference between ruo and ivd.
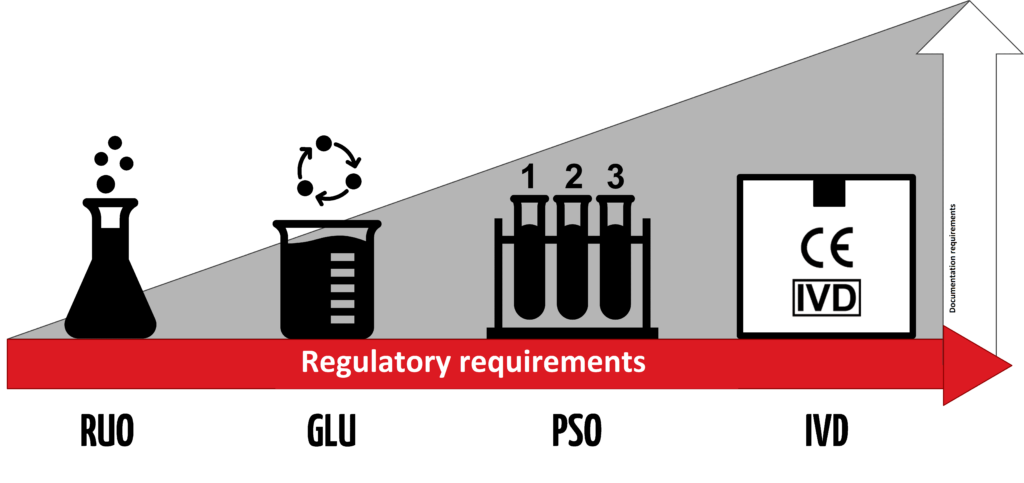
An IVD is an “In Vitro Diagnostic Medical Device,” and the general term applies to any device or product that either alone or with other products is intended to be used for diagnostic, monitoring, or compatibility purposes. There are four different regulatory levels for IVDs:
- Research Use Only (RUO)
- General Laboratory Use (GLU)
- For Performance Studies Only (PSO)
- In Vitro Diagnostic Medical Device (IVD)
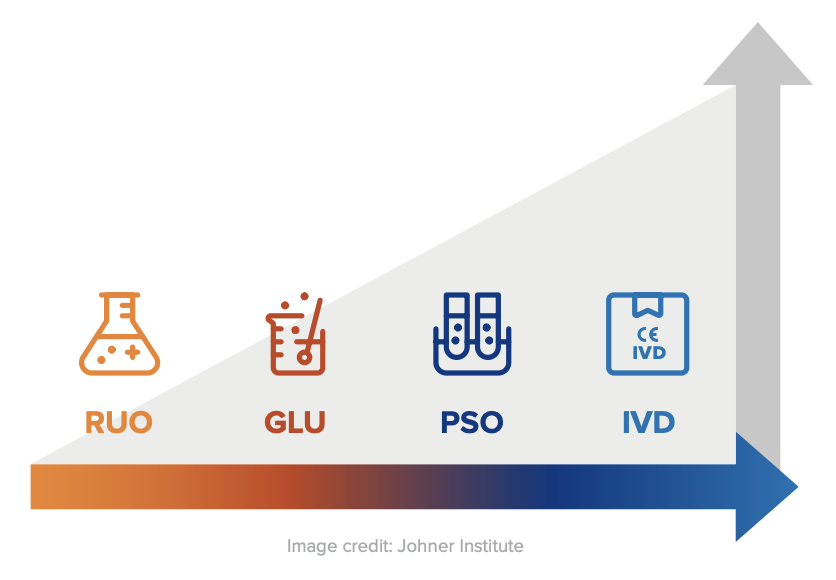
The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest level of regulation, and In Vitro Diagnostic Medical Devices are at the highest level. Occasionally in the US, products will be labeled as “RUO IVD,” which means an in vitro device that is intended for research use only.
Products labeled with the “CE-IVD” label indicate that they have progressed through the applicable regulatory process and standards (such as IVDD or IVDR). These products are approved for diagnostic use and must include the IVD symbol to be used for medical purposes.
In the EU, as of May 2022, IVDs must comply with Regulation (EU) 2017/746 (IVDR) . The IVDR defines IVDs as follows:
“‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state; (b) concerning congenital physical or mental impairments; (c) concerning the predisposition to a medical condition or a disease; (d) to determine the safety and compatibility with potential recipients; (e) to predict treatment response or reactions; (f) to define or monitoring therapeutic measures.”
All IVDs that comply with the IVDR must carry the CE Mark if marketed in the EU.
Research Use Only products are not subject to regulatory requirements in either the US or the EU, but because they don’t meet the same compliance standards as IVDs, they must be clearly labeled as RUO products and cannot be used for medical purposes.
A known exception is the lab developed test (LDT) pathway for clinical purposes.
What are the requirements for an RUO product?
In the US, RUO products are basically unregulated and do not need to meet any specific requirements to carry the RUO label. The FDA does not specify any restrictions or limitations on RUO products, provided they are clearly labeled “For Research Use Only. Not for use in diagnostic procedures.” For this reason, RUO products can be an excellent solution for laboratories that need research materials for testing and research purposes. Because products with the RUO label do not require extensive testing, verification, and validation, they tend to be more cost-effective for research purposes.
The EU rules are similar. Because RUO products do not have clinical applications, they are not considered medical devices, and there are no requirements for RUO products defined by either the IVDD or the IVDR. These products should not be marked with the IVD mark, and they should be clearly labeled as “Research Use Only.”
RELATED: See how Jama Software ® helped Össur improve the mobility of millions by replacing process rigidity with speed and agility.
Are there alternatives to ruo labels.
Given the significant differences between labeling a product as RUO and labeling a product as IVD, manufacturers and users can’t be too careful when it comes to assigning labels or using products for specific purposes. If there is a risk to using products labeled as RUO, manufacturers and users should opt for products that have attained a higher compliance level. For example, for a doctor’s office or home use, IVD is the right path. For clinical purposes or hospital labs, RUO could be used as LDT as long as they are CAP/CLIA certified, such was the case with COVID-19 testing kits when the pandemic first hit.
For products that meet a higher degree of compliance, it is possible to assign General Laboratory Use (GLU), Performance Studies Only (PSO), or even In Vitro Diagnostic Medical Device (IVD) labels. However, depending on the intended use for the Research Use Only products, pursuing these additional levels of compliance may or may not make sense.
What is CLIA certification?
CLIA stands for Clinical Laboratory Improvement Amendments. The Centers for Medicare & Medicaid Services (CMS) regulates all clinical laboratory testing performed on humans in the United States through CLIA.
What is a CAP accreditation?
CAP stands for The College of American Pathologists (CAP) . The purpose of CAP laboratory accreditation is to ensure laboratories provide precise test results for accurate patient diagnoses, meet CLIA and CAP requirements, and demonstrate compliance with professionally and scientifically sound and approved laboratory operating standards.
What are RUO products used for?
As the name implies, RUO projects should be used for research purposes only. They may be used for basic research, pharmaceutical research, or in-house manufacturing of “home brew kits” for research purposes and potentially for clinical applications via the LDT pathway. RUO products are specifically not to be used to make diagnoses, conduct performance studies, or as a substitute or comparator for a CE-IVD device. They may also not be used for market or feasibility studies. Raw ingredients labeled as RUO products may not be incorporated into a finished IVD product.
Learn more about the advantages and disadvantages of the RUO label (and more) by downloading the entire eBook HERE .
- Recent Posts
- [Webinar Recap] Key Systems Engineering Skills: Critical Thinking and Problem Framing - March 5, 2024
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 2 - July 28, 2023
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 1 - July 21, 2023
USA 135 SW Taylor Suite 200 Portland, Oregon, 97204
EUROPE Amsterdam Queens Tower Delflandlaan 1, 1062EA Amsterdam The Netherlands
© 2024 Jama Software
- JAMA CONNECT
- Product Overview
- Pricing and Licensing
- Why Jama Software®?
- Success Programs
- Education & Support
- Resource Library
- Discovery Center
- Guide to Requirements Management
- User Community
- Privacy Policy
- Privacy and Security
- Preferences
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Device Advice: Comprehensive Regulatory Assistance
- Overview of Device Regulation
- Device Labeling
In Vitro Diagnostic Device Labeling Requirements
On this page: , introduction.
- Label Requirements for the Immediate Container
- Labeling Requirements for Inserts and Outer packaging
Exemptions from Labeling Requirements
- Specific labeling requirements for analyte specific reagents
- Restrictions on sale distribution and use of analyte specific reagents
- Specific labeling requirements for OTC test sample collection systems
- Restrictions on the sale, distribution and use of OTC test sample collection systems
In vitro diagnostic products (IVD's) are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body. In vitro diagnostic (IVD) labeling requirements are located in 21 CFR Part 809. Numbers appearing in parentheses next to subject headings are the corresponding sections of 21 CFR. This section contains the basis requirements for label and labeling (package insert) as specified in the labeling regulations for in vitro diagnostic products.
Label Requirements for the Immediate Container [ 21 CFR 809.10 (a)]
The label for IVD's must state the following information, except in cases where it is not applicable. In addition, all information must appear on the outside container or wrapper, or be easily legible through the outside container or wrapper.
If the presence of any label information will interfere with the test, the information may appear on the outside wrapper or container instead of the label.
If the immediate containers are too small, or otherwise unable to bear labels with sufficient space, then the required labeling as listed below annotated with an asterisk (*) may appear on the outer container labeling only.
Label requirements are as follows:
- The established and proprietary names of the product, e.g., cholesterol meters;
- * The intended use or uses, e.g., pregnancy detection, diabetes screening, etc.;
- A statement of warnings or precautions for users listed in 16 CFR part 1500 (hazardous substances) and any other warnings appropriate to user hazards, and a statement "For In Vitro Diagnostic Use;"
- Name and place of business of the manufacturer, packer, or distributor;
- - Multiple unit products must have traceability of the individual units;
- - Instrument lot numbers must allow for traceability of subassemblies; and
- - A multiple unit product that requires use of its components as a system should have the same lot number, or other suitable uniform identification, on all units.
- - Established (common or usual) name;
- - Quantity, proportion, or concentration of all active ingredients; e.g., mg., weight per unit volume, mg./dl etc., and for reagents derived from biological materials the source and measure of its activity, e.g., bovine, I.U., etc.;
- - Storage instructions adequate to protect the stability of the product, i.e., temperature, humidity, etc.;
- - Instructions for manipulation of products requiring mixing or reconstitution, along with instructions for storage of products that have been reconstituted or mixed;
- i. expiration date (date beyond which the product is not to be used);
- * ii. statement of any visual indication of alteration;
- * iii. Instructions for a simple check to assure product usefulness;
- * - The net quantity of contents.
Labeling Requirements for Inserts and Outer Packaging 21 CFR 809.10 (b)
Labeling must contain in one place the following information in the FORMAT and ORDER listed below, except where information is not applicable, or as specified in a standard for a particular product class.
If the device is a reagent intended as a replacement in a diagnostic system, labeling may be limited to that information necessary to adequately identify the reagent and to describe its use in the system.
If the device is a multiple purpose instrument used for diagnostic purposes, and not committed to specific diagnostic procedures or systems, labeling can be restricted to those points annotated by an asterisk (*).
- * The proprietary and established product name;
- * The intended use of the product and whether it is a qualitative or quantitative type of procedure, e.g., screening, physician's office, home use, etc.;
- Summary and explanation of the test, including a short history containing methodology and the special merits and limitations of the test;
- The chemical, physical, physiological, or biological principles of the procedure.
- - The common name, if any, and quantity, proportion, or concentration or each reactive ingredient; and for biological material, the source and measure it its activity;
- - Appropriate cautions or warnings listed in 16 CFR Part 1500; the statement: "For In Vitro Diagnostic Use;" and any other limiting statements appropriate to the intended use of the product;
- - Adequate directions for reconstitution, mixing, dilution, etc.;
- - Appropriate storage instructions;
- - A statement of purification or treatment required for use; and
- - Physical, biological, or chemical indications of instability or deterioration.
- - Use or function;
- - Installation procedures and requirements;
- - Principles of operation;
- - Performance characteristics and specifications;
- - Operating instructions;
- - Calibration procedures, including equipment and/or materials;
- - Operational precautions and limitations;
- - Hazards; and
- - Service and maintenance information
- - Special precautions/preparations;
- - Additives necessary to maintain specimen integrity;
- - Known interfering substances; and
- - Recommended specimen storage, handling, and shipping instructions.
- - A list of materials provided and instruction for use, e.g., reagents, equipment, etc.;
- - A list of necessary materials that are not provided (include details such as sizes, numbers, types, and quality);
- - A description of the amounts of reagents necessary, and parameters such as time, temperature etc.;
- - A statement related to final reaction stability and any time restrictions on accurate measurements;
- - Details of calibration, identifying and listing and necessary preparation of the reference materials, samples, and blanks. Describe the calibration range including the highest and lowest values measured; and
- - Details of necessary quality control procedures and materials, e.g., positive and negative controls, acceptable performance limits.
- Explanation of the procedure for calculating the unknown, including the definition of each component of the formula, a sample calculation, and the number of significant figures appropriate for the answer;
- Limitations of the procedure, e.g., identify situations which will have an adverse impact on test results. If further testing either more specific or more sensitive, is indicated in all cases where certain results are obtained, the need for the additional test shall be stated;
- Expected values including how the range(s) was established and identify the populations on which it was established;
- Specific performance characteristics as appropriate including accuracy, specificity, precision, and sensitivity;
- * Bibliography;
- * Name and place of business of the manufacturer, packer, or distributor; and
- * Date of issuance of the last labeling revision by the firm.
Shipments or other deliveries of IVD devices are exempt from label and labeling requirements in the above headings and from standards listed under Part 861 provided the following conditions are met:
- A shipment or delivery for an investigation subject to Part 812, Investigational Device Exemption (IDE), if the device is in compliance with the subject IDE; or
- - A product in the laboratory research phase, not represented as an IVD, that is prominently labeled: "For Research Use Only. Not for use in diagnostic procedures;" and
- - A product that is being shipped or delivered for product testing prior to full commercial marketing that is prominently labeled: "For Investigational Use Only. The performance characteristics of this product have not been established."
Labeling of General Purpose Reagents and Equipment
General purpose items include routine laboratory reagents such as hydrochloric acid and equipment such as glassware whose uses are generally known by persons trained in their use. They do not need to bear the directions for use listed under Label Requirements for the Immediate Container and Labeling Requirements for Inserts and Outer Packaging, if their labeling meets the requirements listed below. If the product packaging is too small to accommodate a label with sufficient space for the labeling, and if the product is packaged in an outer container which has all of following on its labeling, then only those portions annotated with an asterisk (*) must be on the product label.
- * - A declaration of the established name, if any, and quantity, proportion, or concentration of the reagent ingredient stated in a system generally recognized by the user;
- - A statement of the purity and quality including a qualitative statement of any impurities. This can be satisfied by using a statement of conformity with a generally recognized and available standard;
- - A statement of warnings or precautions for users as contained in the regulations in 16 CFR Part 1500 and any other appropriate warnings, and the statement: "For Laboratory Use;"
- - Net quantity of contents in terms of weight or volume, or numerical count, or any combination thereof;
- * - Name and place of business of the manufacturer, packer, or distributor;
- * - A lot or control number traceable to the manufacturing history of the product; and.
- - A statement indicating the presence of and characterizing any catalytic or nonreactive ingredients e.g., buffers, preservatives, stabilizers.
- - Product labeling need include only a statement adequately describing the product, its composition, and physical characteristics if necessary for its proper use.
Other Resources

- En Español

- Medical Devices
- Radiation-Emitting Products
- Vaccines, Blood & Biologics
- Animal & Veterinary
- Tobacco Products
CFR - Code of Federal Regulations Title 21
The information on this page is current as of Mar 22, 2024 .
For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR).
| [Code of Federal Regulations] |
| [Title 21, Volume 8] |
| [CITE: 21CFR809.10] |





IMAGES
VIDEO
COMMENTS
Regulatory Requirements for Research Use Only and Investigational Use Only IVD products Section 520(g) of the FD&C Act, 21 U.S.C. 360j(g), provides for the exemption of devices
Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs).
Provides the current thinking of CDRH and CBER on when IVD products are properly labeled 'for research use only' (RUO) or 'for investigational use only' (IUO).
Research Use Only (RUO) RUO stands for Research Use Only. The RUO label serves as a warning to clinical laboratory professionals that the materials in question are not intended for use with patient diagnostics.
Research Use Only products (RUOs) can be used in various ways for research purposes. Below are some examples: Fundamental Research. RUO products can be used for fundamental research in the laboratories to understand various aspects of the human body.
This introduction will provide an overview of the Research Use Only label, how it differs from similar, adjacent labels, its appropriate use, and the consequences of mislabeling products RUO.
This section contains the basis requirements for label and labeling (package insert) as specified in the labeling regulations for in vitro diagnostic products.
The guidance clarified the regulatory requirements applicable to in vitro diagnostic (IVD) products intended for research use only (RUO) or investigational use only (IUO). In addition, the guidance serves as a warning that products so labeled should not be used in clinical diagnosis or patient management.
(i) For a product in the laboratory research phase of development, and not represented as an effective in vitro diagnostic product, all labeling bears the statement, prominently placed: "For...
In sharp contrast, research use only (RUO) products are essentially unregulated. In fact, although RUO products are often discussed as though they are a kind of medical device, RUOs are not...