- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes
FDA News Release
Today, the U.S. Food and Drug Administration approved Lantidra, the first allogeneic (donor) pancreatic islet cellular therapy made from deceased donor pancreatic cells for the treatment of type 1 diabetes. Lantidra is approved for the treatment of adults with type 1 diabetes who are unable to approach target glycated hemoglobin (average blood glucose levels) because of current repeated episodes of severe hypoglycemia (low blood sugar) despite intensive diabetes management and education.
“Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 diabetes and recurrent severe hypoglycemia an additional treatment option to help achieve target blood glucose levels.”
Type 1 diabetes is a chronic autoimmune disease that requires lifelong care including requiring insulin, either through multiple daily injections or continuous infusion using a pump, every day to live. People with type 1 diabetes also perform blood glucose checks several times a day to guide the management of their diabetes.
Some people with type 1 diabetes have trouble managing the amount of insulin needed every day to prevent hyperglycemia (high blood sugar) without causing hypoglycemia. They may also develop hypoglycemia unawareness, where they are unable to detect their blood glucose is dropping and may not have a chance to treat themselves to prevent their blood glucose from further dropping. This makes it difficult to dose insulin. Lantidra provides a potential treatment option for these patients.
The primary mechanism of action of Lantidra is believed to be the secretion of insulin by the infused allogeneic islet beta cells. In some patients with type 1 diabetes, these infused cells can produce enough insulin, so the patient no longer needs to take insulin (by injections or pump) to control their blood sugar levels. Lantidra is administered as a single infusion into the hepatic (liver) portal vein. An additional infusion of Lantidra may be performed depending on the patient’s response to the initial dose.
The safety and effectiveness of Lantidra was evaluated in two non-randomized, single-arm studies in which a total of 30 participants with type 1 diabetes and hypoglycemic unawareness received at least one infusion and a maximum of three infusions. Overall, 21 participants did not need to take insulin for a year or more, with 11 participants not needing insulin for one to five years and 10 participants not needing insulin for more than five years. Five participants did not achieve any days of insulin independence.
Adverse reactions associated with Lantidra varied with each participant depending on the number of infusions they received and the length of time they were followed and may not reflect the rates observed in practice The most common adverse reactions included nausea, fatigue, anemia, diarrhea and abdominal pain. A majority of participants experienced at least one serious adverse reaction related to the procedure for infusing Lantidra into the hepatic portal vein and the use of immunosuppressive medications needed to maintain the islet cell viability. Some serious adverse reactions required discontinuation of immunosuppressive medications, which resulted in the loss of islet cell function and insulin independence. These adverse events should be considered when assessing the benefits and risks of Lantidra for each patient. Lantidra is approved with patient-directed labeling to inform patients with type 1 diabetes about benefits and risks of Lantidra.
The FDA granted approval of Lantidra to CellTrans Inc.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
Parkinson’s may take a ‘gut-first’ path

New AI tool can diagnose cancer, guide treatment, predict patient survival
Blood test can warn women of risk decades before heart attack, stroke

“When my son was diagnosed [with Type 1], I knew nothing about diabetes. I changed my research focus, thinking, as any parent would, ‘What am I going to do about this?’” says Douglas Melton.
Kris Snibbe/Harvard Staff Photographer
Breakthrough within reach for diabetes scientist and patients nearest to his heart
Harvard Correspondent
100 years after discovery of insulin, replacement therapy represents ‘a new kind of medicine,’ says Stem Cell Institute co-director Douglas Melton, whose children inspired his research
When Vertex Pharmaceuticals announced last month that its investigational stem-cell-derived replacement therapy was, in conjunction with immunosuppressive therapy, helping the first patient in a Phase 1/2 clinical trial robustly reproduce his or her own fully differentiated pancreatic islet cells, the cells that produce insulin, the news was hailed as a potential breakthrough for the treatment of Type 1 diabetes. For Harvard Stem Cell Institute Co-Director and Xander University Professor Douglas Melton, whose lab pioneered the science behind the therapy, the trial marked the most recent turning point in a decades-long effort to understand and treat the disease. In a conversation with the Gazette, Melton discussed the science behind the advance, the challenges ahead, and the personal side of his research. The interview was edited for clarity and length.
Douglas Melton
GAZETTE: What is the significance of the Vertex trial?
MELTON: The first major change in the treatment of Type 1 diabetes was probably the discovery of insulin in 1920. Now it’s 100 years later and if this works, it’s going to change the medical treatment for people with diabetes. Instead of injecting insulin, patients will get cells that will be their own insulin factories. It’s a new kind of medicine.
GAZETTE: Would you walk us through the approach?
MELTON: Nearly two decades ago we had the idea that we could use embryonic stem cells to make functional pancreatic islets for diabetics. When we first started, we had to try to figure out how the islets in a person’s pancreas replenished. Blood, for example, is replenished routinely by a blood stem cell. So, if you go give blood at a blood drive, your body makes more blood. But we showed in mice that that is not true for the pancreatic islets. Once they’re removed or killed, the adult body has no capacity to make new ones.
So the first important “a-ha” moment was to demonstrate that there was no capacity in an adult to make new islets. That moved us to another source of new material: stem cells. The next important thing, after we overcame the political issues surrounding the use of embryonic stem cells, was to ask: Can we direct the differentiation of stem cells and make them become beta cells? That problem took much longer than I expected — I told my wife it would take five years, but it took closer to 15. The project benefited enormously from undergraduates, graduate students, and postdocs. None of them were here for 15 years of course, but they all worked on different steps.
GAZETTE: What role did the Harvard Stem Cell Institute play?
MELTON: This work absolutely could not have been done using conventional support from the National Institutes of Health. First of all, NIH grants came with severe restrictions and secondly, a long-term project like this doesn’t easily map to the initial grant support they give for a one- to three-year project. I am forever grateful and feel fortunate to have been at a private institution where philanthropy, through the HSCI, wasn’t just helpful, it made all the difference.
I am exceptionally grateful as well to former Harvard President Larry Summers and Steve Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute, who supported the creation of the HSCI, which was formed specifically with the idea to explore the potential of pluripotency stem cells for discovering questions about how development works, how cells are made in our body, and hopefully for finding new treatments or cures for disease. This may be one of the first examples where it’s come to fruition. At the time, the use of embryonic stem cells was quite controversial, and Steve and Larry said that this was precisely the kind of science they wanted to support.
GAZETTE: You were fundamental in starting the Department of Stem Cell and Regenerative Biology. Can you tell us about that?
MELTON: David Scadden and I helped start the department, which lives in two Schools: Harvard Medical School and the Faculty of Arts and Science. This speaks to the unusual formation and intention of the department. I’ve talked a lot about diabetes and islets, but think about all the other tissues and diseases that people suffer from. There are faculty and students in the department working on the heart, nerves, muscle, brain, and other tissues — on all aspects of how the development of a cell and a tissue affects who we are and the course of disease. The department is an exciting one because it’s exploring experimental questions such as: How do you regenerate a limb? The department was founded with the idea that not only should you ask and answer questions about nature, but that one can do so with the intention that the results lead to new treatments for disease. It is a kind of applied biology department.
GAZETTE: This pancreatic islet work was patented by Harvard and then licensed to your biotech company, Semma, which was acquired by Vertex. Can you explain how this reflects your personal connection to the research?
MELTON: Semma is named for my two children, Sam and Emma. Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that’s when I changed my research plan. And my daughter, who’s four years older than my son, became diabetic about 10 years later, when she was 14.
When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs develop. I changed my research focus, thinking, as any parent would, “What am I going to do about this?” Again, I come back to the flexibility of Harvard. Nobody said, “Why are you changing your research plan?”
GAZETTE: What’s next?
MELTON: The stem-cell-derived replacement therapy cells that have been put into this first patient were provided with a class of drugs called immunosuppressants, which depress the patient’s immune system. They have to do this because these cells were not taken from that patient, and so they are not recognized as “self.” Without immunosuppressants, they would be rejected. We want to find a way to make cells by genetic engineering that are not recognized as foreign.
I think this is a solvable problem. Why? When a woman has a baby, that baby has two sets of genes. It has genes from the egg, from the mother, which would be recognized as “self,” but it also has genes from the father, which would be “non-self.” Why does the mother’s body not reject the fetus? If we can figure that out, it will help inform our thinking about what genes to change in our stem cell-derived islets so that they could go into any person. This would be relevant not just to diabetes, but to any cells you wanted to transplant for liver or even heart transplants. It could mean no longer having to worry about immunosuppression.
Share this article
You might like.
Damage to upper GI lining linked to future risk of Parkinson’s disease, says new study
Model uses features of a tumor’s microenvironment across 19 different cancer types
Findings support universal screening of three biomarkers, not just cholesterol
Billions worldwide deficient in essential micronutrients
Inadequate levels carry risk of adverse pregnancy outcomes, blindness
You want to be boss. You probably won’t be good at it.
Study pinpoints two measures that predict effective managers
Weight-loss drug linked to fewer COVID deaths
Large-scale study finds Wegovy reduces risk of heart attack, stroke
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead
Affiliations.
- 1 Department of Nanomedicine, Houston Methodist Research Institute, Houston, TX, USA. [email protected].
- 2 Department of Surgery, Houston Methodist Hospital, Houston, TX, USA. [email protected].
- 3 Department of Radiation Oncology, Houston Methodist Hospital, Houston, TX, USA. [email protected].
- 4 Alberta Diabetes Institute, University of Alberta, Edmonton, Alberta, Canada.
- 5 Department of Surgery, University of Alberta, Edmonton, Alberta, Canada.
- 6 Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, FL, USA.
- 7 Department of Biomedical Engineering, University of Miami, Miami, FL, USA.
- 8 Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
- 9 Department of Microbiology and Immunology, University of Miami Miller School of Medicine, Miami, FL, USA.
- 10 Woodruff School of Mechanical Engineering and Petit Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA, USA.
- 11 J. Crayton Pruitt Family Department of Biomedical Engineering, Herbert Wertheim College of Engineering, University of Florida, Gainesville, FL, USA.
- 12 Diabetes Institute, University of Florida, Gainesville, FL, USA.
- 13 Program in Molecular Medicine, Diabetes Center of Excellence, University of Massachusetts Chan Medical School, Worcester, MA, USA.
- 14 Department of Surgery, The University of Arizona, Tucson, AZ, USA.
- 15 Diabetes Research Institute, IRCCS Ospedale San Raffaele, Milan, Italy.
- 16 Department of Pediatrics, Ellis Fischel Cancer Center, School of Medicine, University of Missouri, Columbia, MO, USA.
- 17 Division of Endocrinology, Metabolism and Lipid Research, Washington University School of Medicine, St. Louis, MO, USA.
- 18 Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO, USA.
- 19 Department of Cardiac Surgery, Boston Children's Hospital, Boston, MA, USA.
- 20 Department of Surgery, Harvard Medical School, Boston, MA, USA.
- 21 Harvard Stem Cell Institute, Cambridge, MA, USA.
- 22 Department of Surgery, University of Minnesota, Minneapolis, MN, USA.
- 23 Department of Veterinary Population Medicine, University of Minnesota, St. Paul, MN, USA.
- 24 Institute of Biomedical Engineering, University of Toronto, Toronto, Ontario, Canada.
- 25 Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canada.
- 26 Department of Biological and Environmental Engineering, Cornell University, Ithaca, NY, USA.
- 27 Department of Bioengineering, Rice University, Houston, TX, USA.
- 28 University of California, San Francisco, Department of Bioengineering and Therapeutic Sciences, San Francisco, CA, USA.
- 29 Brown University, School of Engineering, Providence, RI, USA.
- 30 McEwen Stem Cell Institute, University Health Network, Toronto, ON, Canada.
- 31 Department of Physiology, University of Toronto, Toronto, ON, Canada.
- 32 Advocacy Department, Breakthrough T1D, Washington, DC, USA.
- 33 Department of Medicine, Columbia Center for Translational Immunology, Columbia University, New York, NY, USA.
- 34 Department of Microbiology and Immunology, Columbia University, New York, NY, USA.
- 35 Department of Surgery, Columbia University, New York, NY, USA.
- 36 Department of Pharmacology and Therapeutics, University of Florida, Gainesville, FL, USA.
- 37 UW Health Transplant Center, Madison, WI, USA.
- 38 Division of Transplantation, Department of Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
- 39 Diabetes Center, University of California San Francisco, San Francisco, CA, USA.
- 40 Department of Surgery, University of California San Francisco, San Francisco, CA, US.
- 41 Gladstone Institute of Genomic Immunology, University of California San Francisco, San Francisco, CA, USA.
- 42 Research Department, Breakthrough T1D, New York, NY, USA.
- 43 Research Department, Breakthrough T1D, New York, NY, USA. [email protected].
- 44 Vaccine and Immunotherapy Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. [email protected].
- 45 Immunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Groningen, Netherlands. [email protected].
- PMID: 39227741
- DOI: 10.1038/s41574-024-01029-0
Type 1 diabetes mellitus (T1DM) is a growing global health concern that affects approximately 8.5 million individuals worldwide. T1DM is characterized by an autoimmune destruction of pancreatic β cells, leading to a disruption in glucose homeostasis. Therapeutic intervention for T1DM requires a complex regimen of glycaemic monitoring and the administration of exogenous insulin to regulate blood glucose levels. Advances in continuous glucose monitoring and algorithm-driven insulin delivery devices have improved the quality of life of patients. Despite this, mimicking islet function and complex physiological feedback remains challenging. Pancreatic islet transplantation represents a potential functional cure for T1DM but is hindered by donor scarcity, variability in harvested cells, aggressive immunosuppressive regimens and suboptimal clinical outcomes. Current research is directed towards generating alternative cell sources, improving transplantation methods, and enhancing cell survival without chronic immunosuppression. This Review maps the progress in cell replacement therapies for T1DM and outlines the remaining challenges and future directions. We explore the state-of-the-art strategies for generating replenishable β cells, cell delivery technologies and local targeted immune modulation. Finally, we highlight relevant animal models and the regulatory aspects for advancing these technologies towards clinical deployment.
© 2024. Springer Nature Limited.
PubMed Disclaimer
- International Diabetes Federation. IDF Diabetes Atlas Reports: Type 1 Diabetes Estimates in Children and Adults (International Diabetes Federation, 2022).
- US Centers for Disease Control and Prevention. National Diabetes Statistics Report (CDC, 2024).
- Tonnies, T. et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2060: the SEARCH for diabetes in youth study. Diabetes Care 46, 313–320 (2023). - PubMed - DOI
- Syed, F. Z. Type 1 diabetes mellitus. Ann. Intern. Med. 175, ITC33–ITC48 (2022). - PubMed - DOI
- Ebekozien, O. et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016-2022) from the T1D exchange quality improvement collaborative. Diabetes Technol. Ther. 25, 765–773 (2023). - PubMed - DOI
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Nature Publishing Group

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- New advances in type 1...
New advances in type 1 diabetes
- Related content
- Peer review
In table 3 in this review by Subramanian and colleagues (BMJ 2024;384:e075681, doi: 10.1136/bmj-2023-075681 , published 26 January 2024), the time in range target for most adults with type 1 diabetes has been corrected to >70%. The article and PDF have been updated.

Study provides preliminary evidence in favor of a new type 1 diabetes treatment

Type 1 diabetes is an autoimmune disease that causes the body's immune system to attack and destroy insulin-producing beta cells in the pancreas. Traditional management of type 1 diabetes has primarily involved replacing the missing insulin with injections which, though effective, can be expensive and burdensome. A new study led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment.
The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that plays a key role in cellular metabolism. The latest translational results are a culmination of years of research: In 2010, while corresponding author Raghu Mirmira, MD, PhD , was at Indiana University, he and his lab performed fundamental biochemistry experiments on beta cells in culture. They found that suppressing the metabolic pathway altered by DFMO helped protect the beta cells from environmental factors, hinting at the possibility of preserving and even restoring these vital cells in patients diagnosed with type 1 diabetes.
The researchers confirmed their observations preclinically in zebrafish and then in mice before senior author Linda DiMeglio, MD, MPH, Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a clinical trial to evaluate the safety and tolerability of the drug in type 1 diabetes patients. The results of the trial, which was funded by the Juvenile Diabetes Research Foundation (JDRF) and used DMFO provided by Panbela Therapeutics, indicated that the drug is safe for type 1 diabetes patients and can help keep insulin levels stable by protecting beta cells.
“As a physician-scientist, this is the kind of thing we’ve always strived for – to discover something at a very basic, fundamental level in cells and find a way to bring it into the clinic,” said Mirmira, who is now Professor of Medicine and an endocrinologist at UChicago Medicine. “It definitely underscores the importance of supporting basic science research.”
"It's been truly thrilling to witness the promising results in the pilot trial after this long journey, and we're excited to continue our meaningful collaboration," said DiMeglio.
Importantly, DFMO has already been FDA-approved as a high dose injection since 1990 for treating African Sleeping Sickness and received breakthrough therapy designation for neuroblastoma maintenance therapy after remission in 2020. Pre-existing regulatory approval could potentially facilitate its use in type 1 diabetes, saving effort and expense and getting the treatment to patients sooner.
“For a drug that’s already approved for other indications, the approval timeline can be a matter of years instead of decades once you have solid clinical evidence for safety and efficacy,” said Mirmira. “Using a new formulation of DFMO as a pill allows patients to take it by mouth instead of needing to undergo regular injections, and it has a very favorable side effect profile. It’s exciting to say we have a drug that works differently from every other treatment we have for this disease.”
To follow up on the recently published results, first and co-corresponding author Emily K. Sims, MD, Associate Professor of Pediatrics at IU School of Medicine and a pediatric endocrinologist at Riley Children's Health, launched a multi-center clinical trial, also funded by JDRF – with UChicago among the trial sites – to gather even stronger data regarding the efficacy of DFMO as a type 1 diabetes treatment.
"With our promising early findings, we hold hope that DFMO, possibly as part of a combination therapy, could offer potential benefits to preserve insulin secretion in individuals with recent-onset type 1 diabetes and ultimately also be tested in those who are at risk of developing the condition," said Sims.
“A new era is dawning where we’re thinking of novel ways to modify the disease using different types of drugs and targets that we didn’t classically think of in type 1 diabetes treatment,” said Mirmira.
The study, “Inhibition of Polyamine Biosynthesis Preserves β-Cell Function in Type 1 Diabetes,” was published in Cell Medicine Reports in November 2023. Co-authors include Emily K. Sims, Abhishek Kulkarni, Audrey Hull, Stephanie E. Woerner, Susanne Cabrera, Lucy D. Mastrandrea, Batoul Hammoud, Soumyadeep Sarkar, Ernesto S. Nakayasu, Teresa L. Mastracci, Susan M. Perkins, Fangqian Ouyang, Bobbie-Jo Webb-Robertson, Jacob R. Enriquez, Sarah A. Tersey, Carmella Evans-Molina, S. Alice Long, Lori Blanchfield, Eugene W. Gerner, Raghavendra Mirmira, and Linda A. DiMeglio.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 27, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Study unlocks potential breakthrough in type 1 diabetes treatment
by Silvia Cernea Clark, Rice University
For the well over 700 million people around the globe living with type 1 diabetes, getting a host immune system to tolerate the presence of implanted insulin-secreting cells could be life-changing.
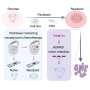
Rice University bioengineer Omid Veiseh and collaborators identified new biomaterial formulations that could help turn the page on type 1 diabetes treatment, opening the door to a more sustainable, long-term, self-regulating way to handle the disease.
To do so, they developed a new screening technique that involves tagging each biomaterial formulation in a library of hundreds with a unique "barcode" before implanting them in live subjects.
According to the study in Nature Biomedical Engineering , using one of the alginate formulations to encapsulate human insulin-secreting islet cells provided long-term blood sugar level control in diabetic mice. Catheters coated with two other high-performing materials did not clog up.
"This work was motivated by a major unmet need," said Veiseh, a Rice assistant professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar. "In type 1 diabetes patients , the body's immune system attacks the insulin-producing cells of the pancreas. As those cells are killed off, the patient loses the ability to regulate their blood glucose."
For decades, scientists labored toward what Veiseh called a "'holy grail" goal of housing islet cells inside a porous matrix made out of a protective material that would allow the cells to access oxygen and nutrients without getting clobbered by the host's immune system.
However, materials with optimal biocompatibility proved very hard to find, due in part to screening constraints. On one hand, immune system response to a given implanted biomaterial can only be assessed in a live host.
"The problem is the immune response needs to be investigated inside the body of these diabetic mice, not in a test tube ," said Boram Kim, a graduate student in the Veiseh lab and co-lead author on the study. "That means that if you want to screen these hundreds of alginate molecules, then you need to have hundreds of animal test subjects. Our idea was to screen for hundreds of biomaterials at the same time, in the same test subject."
On the other hand, different biomaterial formulations look the same, making it impossible to identify high-performing ones in the absence of some telltale trait. This made testing more than one biomaterial per host unfeasible.
"They are different materials but they look the same," Veiseh said. "And once they are implanted in the body of a test subject and then taken out again, we cannot distinguish between the materials and we would be unable to identify which material formulation worked best."
To overcome these constraints, Veiseh and collaborators came up with a way to tag each alginate formulation with a unique 'barcode' that allowed them to identify the ones that performed best.
"We paired each modified biomaterial with human umbilical vein endothelial cells (HUVEC) from a different donor," Kim said.
"The HUVEC cells, because they come from unique donors, act as a barcode that allows us to tell what material was used initially," Veiseh added. "The winners are the ones that have live cells in them. Once we found them, we sequenced the genome of those cells and figured out which material was paired with it. That's how we uncovered the greatest hits."
Trials are underway for stem cell-derived islet cell use in diabetic patients. However, current islet treatments require immunosuppression, making it a taxing way to treat type 1 diabetes.
"Currently, in order to use implanted islet cells in diabetic patients, you have to suppress the entire immune system, just as if you were trying to do an organ transplant," Veiseh said. "That comes with a lot of complications for the patient."
"They can develop cancer, they can't fight infections, so, for the vast majority of patients, it's better to actually do the insulin therapy where they inject themselves. With this biomaterial-encapsulation strategy, no immunosuppression is needed."
Placing actual HUVEC cells inside the biomaterial capsules increased the likelihood that the host immune system would detect a foreign presence. This makes the experiment more robust than simply testing for immune response to the biomaterials alone.
"We wanted to test a library of these materials, with the selection pressure of having cells inside the beads that makes it harder for the material to not get noticed by the immune system," Veiseh said. "There's a lot of interest from all the islet cell manufacturers to be able to get rid of immunosuppression and instead use these alginate hydrogel matrices to protect the implanted cells."
The new high-throughput "barcoding" approach can be deployed to screen for other medical applications using fewer live test subjects.
"That actually feeds into a lot of other projects in my lab where we're doing biologic production from cells for other disease indications," Veiseh said. "The same modifications can be applied to all types of materials that go into the body. This is not limited only to cell transplantation. The technology we developed can be paired with a lot of different device concepts."
"For instance, some diabetic patients use automated pump systems to self-administer insulin. The catheters on those pump systems have to be replaced every few days because they get clogged. We were able to show that coating the catheters with these new materials prevented clogging."
"With this new cell-based barcoding technology, biomaterials research just got an unprecedented boost that will accelerate the translation to clinically applicable products, and make it more affordable," said Dr. José Oberholzer, a transplant surgeon and bioengineer at the University of Virginia.
"This is a real paradigm shift. With this method, we can now screen hundreds of biomaterials at once and select those that the human body does not reject. We can protect cellular grafts from the assaults of the immune system, without the need for immunosuppressive medications," Oberholzer added.
Former Rice bioengineering professor and current NuProbe U.S. CEO David Zhang noted that " high-throughput DNA sequencing has revolutionized many biomedical fields."
"I am pleased to work with Omid to enable the development of improved biomaterials using my team's expertise in DNA sequencing," added Zhang, who was a co-investigator on the grant. "These improved biomaterials can enable durable implanted cell therapies to function as living drug factories, and can have a positively disruptive impact on patients with a variety of chronic diseases."
Explore further
Feedback to editors

Low-impact yoga and exercise found to help older women manage urinary incontinence
6 hours ago

Missouri patient tests positive for bird flu despite no known exposure to animals

Falling for financial scams? It may signal early Alzheimer's disease
19 hours ago

Cognitive behavioral therapy enhances brain circuits to relieve depression
New molecular sensor enables fluorescence imaging for assessing sarcoma severity
22 hours ago
Noninvasive focused ultrasound show potential for combating chronic pain
Study finds TGF-beta and RAS signaling are both required for lung cancer metastasis
23 hours ago
Research team successfully maps the brain-spinal cord connection in humans

Alzheimer's study reveals critical differences in memory loss progression based on the presence of specific proteins
Chemical screen identifies PRMT5 as therapeutic target for paclitaxel-resistant triple-negative breast cancer
Related stories.
Fats can help tag medical implants as friend or foe
Mar 14, 2023

Biomaterial improves islet transplants for treatment of type 1 diabetes
May 13, 2022

Heart attack damage reduced by shielded stem cells
Aug 18, 2020

'Drug factory' implants eliminate ovarian, colorectal cancer in mice
Mar 2, 2022
Beta cell-seeded implant restores insulin production in type 1 diabetes mouse model
Mar 19, 2018

'Drug factory' implants eliminate mesothelioma tumors in mice
Aug 22, 2022
Recommended for you

Researchers develop rapid test to detect dopamine
Sep 5, 2024

Wearable microfluidic band can measure sweat biochemistry during rest or exercise

New AI hair analysis method holds promise for improved health research

Yellow dye solution makes tissue transparent on living animals

Low-carb/high-fat diets for weight loss may actually boost risk of type 2 diabetes
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- News Archive
Large study sheds new light on the complex type 1 diabetes genetics landscape and potential drug targets

In new research, scientists analyzed genetic contributors to type 1 diabetes by studying over 61,000 participants (with and without the disease), including individuals from diverse ancestries. This approach led to the identification of 36 new gene regions associated with type 1 diabetes, some of which are also associated with other autoimmune diseases. Additionally, the scientists did “fine mapping” studies to pinpoint the specific disease-causing variants in genetic regions previously associated with type 1 diabetes, toward elucidating the role these variants may play in the disease process. In another set of experiments, they used their genetic association results with data about other autoimmune diseases to identify 12 genes that could potentially be drug targets for type 1 diabetes. Drugs targeting these genes (by affecting the proteins the genes encode) have been studied in clinical trials for other autoimmune diseases, suggesting they could be repurposed for type 1 diabetes. Some of the targets are already being studied in type 1 diabetes clinical trials of various therapies, but others could potentially be explored in future trials to prevent type 1 diabetes.
This large research study with a diverse population has provided important knowledge about the complex type 1 diabetes genetics landscape, revealing new genetic regions associated with the disease, shedding light on how some genetic variants may influence disease, and identifying potential drug targets. Understanding what genes play a role in type 1 diabetes, and what role they play, paves the way to identify new targets to prevent or treat this autoimmune disease.
Robertson CC, Inshaw JRJ, Onengut-Gumuscu S,…Rich SS. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes . Nat Genet 53: 962-971, 2021.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

New and Emerging Technologies in Type 1 Diabetes
There has been a rapid advancement in the pace of development of new diabetes technologies and therapies for the management of type 1 diabetes over the past decade. The Diabetes Control and Complications Trial conclusively established that tight glycemic control with intensive insulin therapy decreases the rates of diabetes complications in proportion to glycemic control, and diabetes technologies have accordingly been developed to help patients reach these goals. In this review, the authors discuss new diabetes therapeutics and technologies, including new insulin analogues, insulin pumps, continuous glucose monitoring systems, and automated insulin delivery systems.”
- • Innovative and novel technologies for the management of type 1 diabetes hold promise for improving glycemia, decreasing burden of disease management, and improving long-term outcomes.
- • Improvements in the accuracy of real-time continuous glucose monitoring (CGM) have allowed for the development of automated insulin delivery systems that can adjust insulin delivery based on CGM glucose input.
- • The development of new drugs, such as ultrarapid-acting insulins that better mimic physiologic insulin secretion, may lead to improved postprandial glycemia. The advent of stable glucagon formulations may allow for development of dual-hormone closed-loop systems that could further improve glycemic regulation.
New technologies in type 1 diabetes
Intensive insulin therapy for the management of type 1 diabetes (T1D) was established as the standard of care based on the results of the Diabetes Control and Complication Trial (DCCT), which conclusively demonstrated the benefits of tight glycemic control. 1 However, those who received intensive insulin management were at increased risk for severe hypoglycemia, which can be acutely life threatening and can result in seizures, coma, or death. Based on DCCT and other data, the American Diabetes Association (ADA) recommends glycosylated hemoglobin (HbA1c) less than 7% in adults, and recently also in many children and adolescents, in order to decrease the risk of both macrovascular and microvascular complications. 2 To achieve these recommended glycemic targets, patients must monitor blood glucose multiple times a day, closely estimate carbohydrate intake to calculate appropriate meal coverage, and administer multiple doses of insulin, which can have varying effects based on several physiologic factors such as physical activity, illness, or stress. This program results in a significant burden of disease management. Recently published data from the T1D Exchange, which includes more than 22,000 children and adults in the United States, show that less than a quarter of patients with T1D are meeting HbA1c goals. 3 Diabetes technologies are being developed to help decrease disease burden and improve glycemic outcomes. In this article, the authors highlight diabetes technology and therapies including new insulin analogues, continuous glucose monitoring systems (CGM), continuous subcutaneous insulin infusion (insulin pump therapy), as well as automated insulin delivery (AID) systems that integrate CGM and insulin pump technology with mathematical algorithms that automatically adjust insulin delivery ( Box 1 ).
Box 1
Key definitions.
| Real-time continuous glucose monitoring (CGM) | Wearable technology that provides real-time continuous glucose measurements with the options of alerts for hyperglycemia, hypoglycemia, or projected glucose out of target ranges |
| Flash glucose monitoring (FGM) | Glucose monitoring system in which data are stored in a wearable sensor and obtained by scanning the sensor with dedicated receiver or smartphone |
| Automated insulin delivery, artificial pancreas system, closed-loop system, bionic pancreas | Terms that refer to an insulin delivery system that uses mathematical algorithms that can adjust insulin delivery based on CGM input |
| Threshold suspend | Automated insulin suspension when glucose level drops less than a specified threshold |
| Predictive low glucose suspend | Automated insulin suspension when glucose level is predicted to be less than a specified glucose threshold (eg, 70 mg/dL) in a specific period of time (eg, 30 min) |
| Hybrid-closed loop system | An automated insulin delivery system that modulates insulin delivery but still requires quantitative announcement of carbohydrate intake by the user |
| Fully closed-loop system | Automated insulin delivery not dependent on user input |
| Bihormonal (dual hormone) system | An artificial pancreas technology that uses insulin plus an additional hormone (eg, glucagon) intended to achieve better glycemic control than possible with an insulin-only system |
Glucose monitoring
Self-monitoring of blood glucose (SMBG) with finger-stick glucose (FSG) concentrations has become a key component of diabetes care. The ability to obtain a blood glucose measurement and adjust therapy accordingly is a mainstay of treatment to reach glucose targets and prevent hypoglycemia. Glucometer accuracy has increased throughout the years, but not all meters available on the market today meet standards set forth by the Food and Drug Administration (FDA) and International Organization for Standardization. 4 Identifying glucose trends and patterns based on SMBG to make insulin adjustments had been the standard of care set forth by the DCCT, and increased frequency of SMBG is associated with improved glycemic control. 5 Some newer glucometers are Bluetooth enabled and can pair with smartphone applications for patients to better track and identify patterns. 6 However, FSG has limitations in that they provide only an instantaneous snapshot in time of current glucose and do not provide information on glucose trends or direction of change.
CGM and FGM devices measure interstitial glucose and estimate plasma glucose every 5 to 15 minutes, depending on the system. Real-time CGM systems (Dexcom G6, Senseonics Eversense, Medtronic Guardian) actively transmit glucose information to a dedicated receiver, insulin pump, smartphone/watch, and to a cloud network if desired and can provide real-time information to the user regarding (1) rate of glucose change, (2) hyperglycemia and hypoglycemia based on individualized thresholds, and (3) impending hypoglycemia alarms based on glucose trends. The glucose measurements can also be shared by patients with others, such as family members, in real-time for an added degree of security. In the only currently available FGM system (Abbott Freestyle Libre), data are stored within the sensor and can be obtained by scanning the device with dedicated receiver or smartphone. Of note, the next-generation Freestyle Libre 2 CGM recently approved by the FDA is capable of “pushing” optional real-time threshold alerts to a receiver or smartphone. Both CGM and FGM devices can be used in blinded mode to record glucose data on the device for later analysis of glycemic patterns to assist health care professionals in making therapeutic decisions.
Externally worn CGM (Dexcom G6, Medtronic Guardian) and FGM (Abbott Freestyle Libre) devices measure interstitial glucose via a transcutaneous sensor, a filament placed in the subcutaneous tissue connected to an overlying transmitter. More recently a CGM with an implantable sensor system with an externally worn transmitter, the Senseonics Eversense, has been approved for 3 or 6 months of use before replacement in the United States and Europe, respectively. Some devices require regular calibration, with FSG input required at least twice daily (Medtronic Guardian and Senseonics Eversense), or are factory calibrated with no additional measurements required (Dexcom G6 and Abbott Freestyle Libre).
Data from CGM and FGM devices can be downloaded by clinicians and provide a standardized ambulatory glucose profile with information regarding percentage of time spent in hypo- and hyperglycemic ranges, time in target range, and glucose variability. Mean glucose as determined by CGM can be used to calculate the glucose management indicator, which provides an estimate of HbA1c 7 to help determine if patients are achieving target glucose goals. 8 In fact, because the relationship between HbA1c and average glucose can be modified by the mean red blood cell lifespan, mean CGM glucose may be a better predictor of long-term complications than HbA1c when the measured HbA1c and GMI are not in agreement. 7 Recently the ADA has published consensus guidelines regarding the recommended percentage of time in target range as well as hyper- and hypoglycemic targets for patients with T1D. 9 Time in target range of 70 to 180 mg/dL (TIR) has been shown to correlate with mean glucose and HbA1c. TIR of 70% correlates to an HbA1c of approximately 7%. TIR has been suggested as a new treatment standard based on the argument that TIR is easier for people with diabetes to understand and is more actionable on a day-to-day basis. 2 , 10 Targets for time below range (TBR) and time above range (TAR) have also been established ( Table 1 ).
Table 1
Continuous glucose monitoring recommendations for patients with type 1 diabetes to achieve HbA1c 7% a
| Glycemic Target (mg/dL) | % of CGM Readings | |
|---|---|---|
| Time below range (TBR) | <54 | <1% |
| <70 | <4% | |
| Time in range (TIR) | 70–180 | >70% |
| Time above range (TAR) | >180 | <25% |
| >250 | <5% |
CGM accuracy has improved significantly since its inception, and many CGM devices have obtained approval for nonadjunctive use (Dexcom G6, Senseonics Eversense, Freestyle Libre), meaning that CGM data can be used as a replacement for FSG when making insulin-dosing decisions. 11 Studies have shown that CGM use is associated with improved HbA1c and a reduction in hypoglycemia. 12 , 13 More recently, the FDA has created an interoperable integrated continuous glucose monitoring system standard, which allows an approved CGM device to be used as part of an integrated system with other compatible medical devices and electronic interfaces, including insulin delivery systems. Approved systems (currently, the Dexcom G6 and the Freestyle Libre 2) meet accuracy and reliability standards set forth by the FDA, securely transmit glucose data to other devices, and may be used interchangeably with AID devices for the purpose of managing glycemia.
One of the major challenges to managing glycemia in patients with diabetes is the inability of currently available insulin formulations to mimic the kinetics and action of endogenous insulin secretion. 14 In individuals without diabetes, incretin-stimulated insulin release and a rapid hepatic exposure to insulin in response to a meal occur and lead to decreased hepatic glucose production. 15 This physiology is no longer intact in patients with T1D. Exogenous insulin administered in the subcutaneous tissue takes time to be absorbed in the systemic circulation. This delayed systemic delivery of exogenous insulin is a major physiologic difference with the immediate entry of endogenous insulin into the hepatic circulation for rapid effects. 16
Since the discovery of insulin in 1921, insulin therapy has greatly advanced from porcine and bovine insulin derivatives to the development of rapid-acting, and then ultrarapid-acting, insulin analogues. Older insulins such as Neutral Protamine Hagedorn and regular human insulin have a slow action of onset and long duration, which require patients to have rigid food consumption timing and routines to match the kinetics of insulin action. Rapid-acting insulin analogues (aspart, lispro, and glulisine) have a faster onset of action and quicker time to peak insulin action, which help better match postprandial glucose excursion. These rapid-acting insulins permit greater flexibility for patients: doses can be adjusted based on the timing and quantity of carbohydrates consumed rather. However, rapid-acting insulin analogues still require injection 10 to 15 minutes before meal intake for optimal action. 14
New ultrarapid-acting insulins have even faster on-off kinetics than rapid-acting insulin. 17 , 18 , 19 , 20 , 21 Faster aspart (also known as Fiasp) is currently FDA approved for adults and children with diabetes and uses nicotinamide as an excipient and l -arginine to increase stability. Ultrarapid lispro (URLi), which has recently completed a phase 3 trial, uses treprostinil to promote vasodilation and citrate as an excipient. 21 BioChaperone lispro, which uses BC222, an oligosaccharide modified with natural molecules and citrate as an excipient, is currently in development. Postprandial glucose were found to be lower with use of faster aspart in both pump and MDI delivery. 19 , 22 Overall rates of blood glucose–confirmed hypoglycemia and severe hypoglycemia have been reported to be similar between aspart and faster aspart. 19 Faster aspart is labeled for use to be administered up to 20 minutes after meal, which can provide further flexibility to patients. A trial of URLi in patients with T1D showed decreased postprandial glycemic excursions at 1 and 2 hours compared with lispro. 21 A short-term, cross-over trial comparing BioChaperone Lispro with insulin lispro has also shown decreases in early postprandial hyperglycemia. 20 In a head-to-head study, BioChaperone Lispro had slightly faster on-off kinetics than insulin lispro and may more closely mimic normal postprandial insulin secretion. 17 Inhaled insulin (Afrezza) is FDA approved and has much more rapid kinetics than injectable insulin delivered subcutaneously. Limitations in clinical use include lack of dose equivalency with injectable insulin and possible respiratory side effects including lung function decreases that are reversible on discontinuation. 23
Insulin delivery modalities
Multiple daily injection.
Insulin has been traditionally administered via MDI therapy via insulin syringe or insulin pen. Smart pen technology pairs the insulin pen with a smartphone to allow patients to more easily calculate and track insulin administration. The InPen (Companion Medical) is currently the only FDA-approved smart pen device, although others are in development. The InPen connects with a smartphone app via Bluetooth allowing patients to track insulin dosing history, calculate insulin doses, keep track of “insulin on board” (an estimate of rapid-acting insulin still in effect) and adjust calculated dosing accordingly, and set dosing reminders. 24 In addition, the phone application can also receive CGM data directly and in real time. Patients can export data collected from the application and share it with their health care team. Smart pen technology may have extra utility in certain patient populations or clinical scenarios, such as those who have difficulty remembering insulin dosing (eg, pediatric patients or those with cognitive or memory impairment) or those with limited health numeracy. 25 Accurate tracking of insulin dose administration is also of use to treatment teams to aid in insulin regimen adjustments. Further research is needed to determine clinical benefits of this technology, and other companies (including major insulin manufacturers) have announced plans to release smart pens in the future.
Insulin Pumps
Insulin pumps deliver a continuous infusion of insulin via a cannula placed in the subcutaneous tissue, sometimes referred to as continuous subcutaneous insulin infusion (CSII). Most of the pumps available use an infusion set with tubing to deliver insulin (in the United States, pumps from Tandem and Medtronic), but some systems known as patch pumps attach directly to the skin without the need for tubing (in the United States, the Insulet Omnipod system). Insulin pumps have programmable basal and bolus settings that can vary based on the time of the day. Insulin pumps track insulin usage and contain bolus calculators to assist in the calculation of meal-time insulin coverage and glucose correction. The pump also keeps track of “insulin on board” and adjusts calculated doses accordingly. The abilities to use different basal rates at different times of the day, to make temporary basal rate adjustments in response to glucose trend or activity level, and to deliver meal-time bolus insulin over extended periods of time based on user input are all unique to insulin pumps.
Patients can achieve target HbA1c goals with either MDI or insulin pump therapy, and extensive research has sought to determine if glycemic control with pump therapy is superior to that of MDI management. A systematic review and meta-analysis showed that both MDI and pump therapy resulted in comparable levels of glycemic control and incidence of severe hypoglycemia in children and adolescents with T1D and that pump therapy may have favorable effects on glycemic control in adults with T1D. 26 Insulin pump therapy is also associated with improved quality of life in both pediatric and adult populations. 27 , 28 By allowing varied basal rates, insulin pumps permit more flexible and physiologic insulin delivery that can be changed based on time of day and other factors such as exercise, as well as varied delivery of meal-time insulin bolus (eg, dual-wave or square-wave delivery set by the user) based on the type of food consumed. In addition, pump therapy eliminates the need for multiple daily injections of insulin, instead requiring only infusion set be changed every 2 to 3 days. Uptake of CSII has increased over the past decade, and currently nearly half of all patients with T1D in the United States manage their diabetes with pump therapy. 3
Automated Insulin Delivery
AID systems (also known as closed-loop, artificial pancreas, or bionic pancreas systems) use real-time glucose measurements fed into a control algorithm that automatically adjusts the rate of subcutaneous insulin delivery via an insulin pump ( Table 2 ). The earliest approved AID systems used threshold suspend, in which insulin delivery way automatically suspended when blood glucose level dropped less than a certain threshold. 29 Predictive glucose suspend improves on this feature by suspending insulin delivery when a hypoglycemic event is predicted in the future. Predictive low glucose suspend functionality decreases the percentage of time spent in hypoglycemic ranges in both the daytime and overnight. 30 By suspending insulin before a hypoglycemic event, this feature also reduces the duration of hypoglycemic events when they do occur.
Table 2
Current Food and Drug Administration–approved automated insulin delivery systems
| MiniMed 530G (Medtronic) | MiniMed 630G Pump (Medtronic) | Basal-IQ System (Tandem) | MiniMed 670G (Medtronic) | Control IQ System (Tandem) | |
|---|---|---|---|---|---|
| Threshold suspend | Predictive low glucose suspend | Predictive low glucose suspend | Hybrid-closed loop | Hybrid-closed loop |
Later generation AID systems entail more complex algorithms to not only suspend insulin delivery based on hypoglycemia but continuously adjust insulin delivery in response to glycemic trends. The most advanced AID systems that are commercially available today are referred to as hybrid closed-loop systems. Patient input is still required to count carbohydrates and administer correction boluses, but the system will additionally modulate insulin delivery in the background, and in some systems deliver partial correction boluses, based on glycemic trends. Other systems that have been studied but are not yet available use qualitative meal announcements to estimate carbohydrate content, describing meals as “typical,” “more than typical,” “less than typical,” or “a small bite,” rather than requiring quantitative carbohydrate counting. 31
Currently available FDA-approved hybrid closed-loop systems include the Medtronic 670G and Tandem t:slim X2 with Control IQ. The first hybrid closed-loop system available in United States, the Medtronic 670G, was approved in 2017 for adult and pediatric patients as young as age 7 years. The approval relied on a nonrandomized study without a control arm. 32 The system can be used as a traditional pump or in “auto mode,” in which the pump automatically adjusts basal insulin rates up to every 5 minutes by increasing, decreasing, or suspending delivery of insulin based on CGM trends. Patients are still required to count carbohydrates and enter them into the system, and meal boluses are calculated based on a programmed carbohydrate ratio. As a safety feature, the system may exit auto mode and revert to preprogrammed delivery if insulin delivery approaches maximum or minimum insulin delivery thresholds, if POC and CGM readings are discrepant, or if CGM signal is lost. In a real-world, prospective observational study of 92 youth who started this system, 30% discontinued use of the auto mode within the first 6 months. Another real-world cohort study of 79 pediatric and adult patients reported that 33% discontinued auto mode use within 12 months. 33 , 34 Reasons cited included the number of alarms, challenges with requiring calibrations, and dissatisfaction with glycemic control. 34
The second hybrid closed-loop device in the United States, the Tandem t:slim X2 with Control-IQ using the Dexcom G6 as the input CGM, was approved in 2019 for adults and pediatric patients older than or equal to 6 years. In the 6-month, randomized, controlled pivotal trial of this device, participants were randomized to closed-loop control or usual diabetes care with sensor-augmented pump therapy. 35 Patients randomized to closed-loop control had improvements in target range, mean CGM glucose, and HbA1c, as well as reduced rates of hypoglycemia. Unlike the Medtronic 670G, Control-IQ only reverts to preprogrammed insulin delivery when CGM signal is lost and does not require finger-stick calibration to continue AID. Trials are underway evaluating this device in younger children ( NCT03844789 ).
Experimental Automated Insulin Delivery Systems
Several AID systems that rely on different sets of mathematical algorithms, including proportional integral derivative, fuzzy logic, and model predictive control algorithms, are in development. These AID systems have been associated with increased time in target glucose range (typically 70–180 mg/dL) and in some cases with decreased mean glucose, lower HbA1c, and decreased time in the hypoglycemic range. Pivotal trials for several of these AID systems are currently ongoing, including the Omnipod Horizon hybrid closed-loop system ( NCT04196140 ) and the Beta Bionics iLet Bionic Pancreas ( NCT04200313 ).
One class of AID systems, called bihormonal or dual hormone systems, is capable of delivering a second hormone to further improve glycemic control. Given the kinetics of subcutaneous insulin delivery, the reduction and/or suspension of insulin may be insufficient to prevent hypoglycemia, especially in certain scenarios that may result in changes in insulin sensitivity such as exercise. Several bihormonal systems use microdosing of glucagon to prevent and treat hypoglycemia when suspension of insulin delivery is not sufficient. Glucagon has rapid on and off kinetics, and the addition of glucagon can allow for more aggressive glucose targets compared with insulin-only systems by reducing the potential for hypoglycemia. In short-term studies of bihormonal systems, subjects achieved increased time in target range, lower mean glucose, and decreased rates of hypoglycemia compared with sensor-augmented pump therapy. 31 , 36 Additional studies comparing bihormonal with insulin-only closed-loop systems suggest that bihormonal systems may further improve mean glucose, time in range, as well as reduce the time spent in hypoglycemic ranges. 37
Other classes of dual hormone systems that have been studied administer pramlintide (an amylin analogue) or glucagon-like peptide-1 (GLP-1) receptor agonist in combination with insulin. 38 , 39 Amylin is cosecreted with insulin from pancreatic beta cells and helps moderate postprandial glucose excursions by slowing gastric emptying, inhibiting glucagon secretion, and promoting satiety. A recent study examining an automated system delivering fixed dose ratio of insulin and pramlintide found increase in time in range compared with the insulin-only system. 38 Long-term studies of bihormonal systems are needed to establish their potential benefits.
A recent meta-analysis 37 reviewed published studies of artificial pancreas systems including insulin-only and dual hormone systems delivering glucagon in more than 500 adult and pediatric subjects with T1D. Most of these trials were small and for a short duration, but the analyses showed that AID systems achieved higher TIR compared with conventional pump therapy and that dual hormone systems resulted in greater improvements in TIR than insulin-only systems. Both classes of AID systems deliver improved glycemia overnight, which is a substantial benefit to patients, as fear of nocturnal hypoglycemia is a primary concern for patients and families. 37 , 40
Challenges to Fully Automated Insulin Delivery
One the main challenges to achieving fully automated closed-loop insulin delivery is overcoming the kinetics of nonphysiologic subcutaneous insulin administration related to postprandial glucose excursions. Given the kinetics of subcutaneous insulin delivery, increased insulin dosing that occurs only after the glucose excursion has begun may lead to prolonged hyperglycemia. Furthermore, because of variations in physiologic insulin needs and the kinetics of current insulin formulations, increased insulin delivery can result in late hypoglycemia. Exercise can compound these challenges by altering insulin sensitivity and increasing insulin-independent glucose uptake into muscles. Several approaches have been studied to ameliorate this issue. Adjunctive therapies including pramlintide (an amylin analogue), GLP-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium–glucose cotransporter 2 inhibitors have all been studied in patients with T1D with the goal of decreasing postprandial glycemic excursions and reducing the need for aggressive insulin dosing. 41 Alternate approaches to insulin delivery, such as delivery of insulin directly to intraperitoneal space, enable faster pharmacokinetics/pharmacodynamics than subcutaneous insulin delivery. 42 Studies examining the utility of new ultrarapid-acting insulins in AID systems have suggested decreased glycemic variability with these newer insulin analogues. 43
Data management and telehealth
Technology including CGM, smartphones, smartwatches, and activity trackers generate large amounts of high-density data that can be difficult for clinicians to synthesize in the limited time available during visits. At present, SMBG, CGM, and pump data can be downloaded to review for patterns and make adjustments in treatment. Currently available software allows patients to download their pump and CGM at home and then share these data via cloud-based services with the patient’s clinical team to review, potentially allowing for more frequent patient contact between in-person visits. With advancement of artificial intelligence and machine learning, these data could be analyzed for automated generation of recommendations for therapy adjustment. Software systems have been developed to automatically generate insulin dose decision support recommendations. 44
The prevalence of technology at home and in clinics has led to great interest in telehealth—a broad term used to describe health care delivery with the aid of technology, which includes video visits, web-based portals, or text messaging. Telehealth has been applied across multiple specialties and conditions and can be used to conduct remote patient visits and patient education and behavioral management sessions. Telehealth strategies can help increase access to health care and reduce barriers to reaching providers, especially in resource limited settings or for those living far from treatment facilities. A recent meta-analysis found that telehealth intervention in patients with diabetes led to HbA1c improvements. 45 Concerns about spread of SARS-CoV-2 have dramatically increased use of telehealth visits for diabetic patients over a very short period of time in the first quarter of 2020 and will likely accelerate the movement of diabetes management visits to virtual formats.
Availability of data in the cloud has allowed companies to publish “real world” studies describing glycemic control in patients using their technologies. 46 The development of virtual diabetes clinics is likely on the horizon, as patient data are obtained from wearable devices including CGM and insulin pumps and then transmitted into the electronic health record for analysis with machine learning and decision support. 47
Diabetes technology holds promise for improving glycemic outcomes and decreasing burden of disease for patients and families with T1D. Rapid advancement of diabetes therapeutics and technologies have enhanced diabetes monitoring and insulin delivery capabilities. Devices that partially automate insulin delivery improve glycemic control, and more capable automated closed-loop systems will likely be available in the near future. Further research should determine the long-term benefits of these devices on glycemic control and quality of life in T1D.
J.S. Sherwood has nothing to disclose. S.J. Russell has patents and patents pending on aspects of the bionic pancreas that are assigned to Massachusetts General Hospital and are licensed to Beta Bionics, has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia, serves on the scientific advisory boards of Unomedical and Companion Medical, has received consulting fees from Beta Bionics, Novo Nordisk, Senseonics, and Flexion Therapeutics, has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics, and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascencia, Senseonics, Adocia, and Tandem Diabetes. M.S. Putman has nothing to disclose.
Talk to us about diabetes
0345 123 2399
customer support
Your top priorities for research into type 1 diabetes revealed

Hundreds of people with type 1 diabetes, their families and healthcare professionals have chosen their most pressing research priorities for type 1 diabetes. The top ten priorities will help to guide future type 1 diabetes research in the UK and Ireland to make sure it has the greatest possible benefit for people with the condition.
As the UK’s largest charitable funder of diabetes research, it’s critical that our research funds address the specific challenges and needs of people with diabetes and those who care for them.
It’s also critical that we make sure others - academics, healthcare professionals and other research funders – hear these views loud and clear and act upon them.
We work with the Priority Setting Partnership (PSP) initiative, run by the James Lind Alliance (JLA) and supported by the National Institute for Health Research (NIHR) , to help bring the views of people with real-life experience of diabetes into research.
Through surveys and workshops, this initiative finds and prioritises their most pressing concerns and questions that can be answered through research. Diabetes UK contributed to the first Type 1 Diabetes PSP in 2011, the Diabetes and Pregnancy PSP in 2020 , and led the Type 2 Diabetes PSP in 2017.
Your new top ten priorities
Since the last Type 1 Diabetes PSP in 2011 there's been some big changes in type 1 treatment and care , so the priorities were due an update. The latest Type 1 PSP – which condensed and whittled down nearly 3000 questions submitted by people affected by type 1 to a shortlist of the top ten – has just been published . And here they are:
1. Can the use of artificial intelligence or faster acting insulins help achieve fully closed loop insulin delivery?
2. Is time in range a better predictor of diabetes management and complications compared to HbA1c (an average reading of blood sugar over a 3-month period)?
3. What impact do hormonal phases such as the perimenstrual period and menopause play in glycaemic management and what treatments are most effective for managing glucose levels around these times?
4. What interventions are the most effective for reducing diabetes related distress and burnout?
5. What are the long-term implications of frequent hypoglycaemia on physical and mental health?
6. What impact does type 1 diabetes (including frequent low blood sugar) have on memory and cognition in older adults?
7. How can health care professionals better take into account the physical, psychological and social aspects of type 1 diabetes in clinics?
8. How can access to potential therapies like stem cell therapy, transplants and medications that modify the immune systems be improved so that everyone with type 1 diabetes can be guaranteed access?
9. Why do some people with type 1 diabetes become insulin resistant and does resistance increase with the number of years a person has diabetes and if so, why?
10. Can technology assist to accurately count carbohydrates without having to weigh or measure all foods and drink?
Dr Christine Newman, Lead Clinical Researcher at the Health Research Board Diabetes Collaborative Clinical Trial Network in Ireland who funded the PSP, emphasised the importance of these findings:
“This study is a powerful example of how Public and Patient Involvement can shape the future of healthcare. This work highlights the real-world challenges and unmet needs of adults living with Type 1 diabetes. By focusing on these top ten priorities, we can ensure that future research and healthcare services are aligned with what truly matters to those affected by the condition.”
We will use these top 10 research priorities in the decisions it makes about how research is funded, and they will inform the work of the Diabetes Research Steering Groups .
We will also publicise these priorities widely to researchers and organisations that fund diabetes research. The priorities could influence those who work in universities and academic institutions, government agencies or in industry.
Dr Elizabeth Robertson, Director of Research at Diabetes UK, explains:
“We need to make sure research that we fund has the greatest possible benefit for people with diabetes. Knowing the most important priorities of people living with or treating type 1 diabetes will help us direct funding to where it’s needed most.”
Share this Page
© The British Diabetic Association operating as Diabetes UK, a charity registered in England and Wales (no. 215199) and in Scotland (no. SC039136). A company limited by guarantee registered in England and Wales with (no.00339181) and registered office at Wells Lawrence House, 126 Back Church Lane London E1 1FH

Best exercises for people with type 1 diabetes outlined in new research

Daily Pastry Consumption Could Silently Harm the Heart Within a Month
Conor seery, share article.
Researchers have revealed what types of exercise suit people living with type 1 diabetes .
Academics from the Universidade Federal do Vale do São Francisco in partnership with Staffordshire University have outlined that gender determines what exercise an individual with type 1 diabetes should do.
Senior author Dr Pooya Soltani said: “This study is important because people with diabetes often lack motivation to exercise as a means of managing their condition.
“One reason for this is that physical activity can lead to blood sugar drops, causing discomfort and demotivation. We investigated whether the type of physical activity could mitigate these blood sugar drops .”
- Children whose mothers have type 1 diabetes less likely to have condition than if their father does
- Type 1 diabetes among youths with presymptomatic symptoms was higher during pandemic
- Type 1 diabetes risk after age 10 differs between boys and girls
A total of 19 adults with type 1 diabetes took part in the study. The research team looked at each participant’s glycaemic and cardiovascular responses following interval exercise sessions and continuous exercise sessions.
Each person involved in the trial participated in half an hour of moderate aerobic exercise on a treadmill.
Meanwhile, the interval aerobic exercise session included alternating one-minute intervals at 40% and 60% of estimated maximal oxygen consumption (VO 2max ). Whereas the participants performed the continuous exercise session at 50% of VO 2max .
The researchers analysed the participant’s blood pressure, heart rate and blood glucose levels prior to and instantly after the exercise sessions, as well as 20 minutes after.
According to the study, greater blood glucose reductions were seen in men than women after continuous aerobic exercise and interval exercise.
Women only reduced their blood glucose values after continuous exercise, and not interval exercise, the results have reported.
Fellow author Dr Jorge Luiz de Brito-Gomes said: “Our study shows that for male patients, interval exercise, such as short bursts of walking, is preferable when starting with low blood sugar levels.
- Time in Range
- Guide to HbA1c
- Blood Sugar Level Ranges
“Conversely, continuous exercise, like running, is more suitable for those with higher initial blood sugar levels. These approaches can help prevent sudden blood sugar drops.”
Dr Luiz de Brito-Gomes added: “For female patients, both interval and continuous aerobic exercise appear to be effective starting points.
“We hope these findings show that gender-specific recommendations should be considered for aerobic exercise prescription, especially for men with irregular physical activity levels.”
Read the study here .
What's new on the forum? ⭐️
T2 for several years, now told i'm actually t1 type 1 diabetes.
Reading vary every morning Ask A Question

What have you eaten today? (Low carb forum) Low-carb Diet Forum
Administrator
‘Stop Eating After 7pm’ Challenge Type 2 Diabetes
MissMuffett
Weekly weigh in - Fridays Type 2 Diabetes
Fasting Blood Sugar this morning + yesterday's food AND physical activity Type 2 Diabetes
Introduce Yourself: Answer Some Personal Questions Greetings and Introductions
Vitanix glucose watch Ask A Question
Let my guard down. Diabetes Discussions
Nicksellick
BG levels after meals Prediabetes
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.
You May Also Like

- Uncategorised
Public Health England considers low carb approach for type 2 diabetes
- 2 minute read

Coronavirus: UK instructed to stay at home this weekend
- 1 minute read

- Coronavirus
Conversation about doctors’ appointments occurring virtually rumbles on
- 3 minute read
Type 1 Diabetes
Type 1 diabetes mellitus describes a condition where the body cannot produce insulin which leads to a very high level of blood sugar and associated complications. The condition, which usually develops in childhood or adolescence, affects millions of people worldwide.
Diabetes Mellitus Type 1 Pathophysiology
Diabetes mellitus type 1 inheritance, type 1 diabetes treatment, feature articles, type 1 diabetes cure, type 1 diabetes prevention, type 1 diabetes research, does the 'stomach flu' vaccine prevent type i diabetes, latest type 1 diabetes news and research.

Scientists advance type 1 diabetes treatment with cutting-edge stem cell and gene editing technologies
Researchers review significant advancements and ongoing challenges in cell replacement therapies for type 1 diabetes, highlighting innovative approaches in stem cell-derived islets and immune protection strategies.

Funding boost accelerates development of promising rheumatoid arthritis treatment
Long term relief is within reach for people with the debilitating autoimmune disease, Rheumatoid Arthritis (RA), after an $11.5 million grant was awarded to a University of Queensland-led team.

Study reveals bidirectional relationship between diabetes complications and mental health disorders
Heart attack, stroke, nerve damage. These are just some of the complications for which millions of Americans with diabetes are at greater risk.

Understanding gluten's role in autoimmunity and inflammation
The interaction between humans and their environment, mediated by nutrition, plays a crucial role in regulating inflammatory responses.

Colorado's $100 copay cap improves diabetes medication adherence and health outcomes
Researchers examined the relationship between Colorado's $100 copayment maximum and self-expenditure, medication compliance, and health service usage for diabetes-associated comorbidities.
_d2c3d4d55fbf40688068be079265900e-150x125.jpg)
Engineering extracellular vesicles for potential treatment of type 1 diabetes
Within each of us lies an army of cells whose topmost duty is protecting against external pathogens and internal threats such as proliferating cancer cells.
McGill discovery offers hope for targeted stem cell treatments
A new technique developed by McGill researchers for mechanically manipulating stem cells could lead to new stem cell treatments, which have yet to fulfill their therapeutic potential.

Poor physical function, not bone density, drives higher fracture risk in older women with type 2 diabetes
Study indicates that older women with type 2 diabetes have a higher risk of fractures primarily due to impaired physical function rather than reduced bone density, despite stronger bone structure.

COVID-19 linked to higher diabetes risk, vaccination reduces impact
COVID-19 significantly increases the incidence of type 2 diabetes, particularly in unvaccinated individuals and those hospitalized. Vaccination and regular diabetes screening post-COVID-19 are crucial for managing long-term health impacts.

Does type 1 diabetes affect taste and flavor recognition?
Type 1 diabetes patients show reduced taste and flavor perception, affecting food preferences and quality of life. This highlights the need for further research into the sensory changes in T1D.
Research reveals autoimmune mechanism behind multisystem inflammatory syndrome in children
Study reveals a mechanism behind multisystem inflammatory syndrome in children (MIS-C).
_1b78fec41a5143afb6e7b9ad317023d2-150x125.jpg)
State-level insulin cost caps ineffective in increasing insulin use among diabetes patients
In a first-of-its-kind study, a cohort of researchers, led by the University of Colorado Anschutz Medical Campus, evaluated the effects of state-level insulin out-of-pocket costs across states and payers and over time.
FDA clears UAB startup's TIX100 for clinical trials
The University of Alabama at Birmingham startup TIXiMED, Inc., has obtained clearance from the United States Food and Drug Administration to proceed to clinical trials under an Investigational New Drug for TIX100, its novel oral Type 1 diabetes drug.

Study reveals curcumin’s antidepressant effects in obese type 2 diabetes patients
A randomized clinical trial (RCT) to investigate the effectiveness of curcumin in lowering depression among obese individuals with diabetes mellitus type 2 (T2DM).

Xanthones show promise in diabetes with antioxidant and anti-inflammatory properties
Research reveals xanthones as promising agents in diabetes management, offering antioxidant, anti-inflammatory, and insulin-sensitizing benefits.

New study highlights paternal influence on child's T1D risk
New research to be presented at this year's Annual Meeting of the European Association for the Study of Diabetes (EASD) (Madrid, 9-13 September) shows that a child is almost twice as likely to develop type 1 diabetes (T1D) if their father has the condition than if their mother has the condition.
New review highlights advances in diabetes research and treatment
A new paper surveying advances in diabetes pathogenesis and treatment explores the complex factors contributing to the onset and progression of the disease, suggesting that an understanding of these dynamics is key to developing targeted interventions to reduce the risk of developing diabetes and managing its complications.
Study reveals reduced type 1 diabetes risk in post-10-year-old girls
New research presented at this year's Annual Meeting of the European Association for Study of Diabetes (Madrid, Spain, 9-13 September) shows that the risk of developing type 1 diabetes (T1D) decreases markedly in girls after age 10 years, while the risk in boys stays the same.
Novel peptide PEPITEM shows promise in fighting 'inflammaging'
A naturally occurring peptide called PEPITEM could potentially rejuvenate the immune response in older individuals and protect against 'inflammaging', which is widely believed to be the root cause of many age-related diseases.

Type 1 diabetes in children linked to increased risk of mental health problems
Children diagnosed with type 1 diabetes are at significantly higher risk of a number of mental health issues, including mood and anxiety disorders, a study from a team in the UK and the Czech Republic has found.
- Trending Stories
- Latest Interviews
- Top Health Articles

How can microdialysis benefit drug development
Ilona Vuist
In this interview, discover how Charles River uses the power of microdialysis for drug development as well as CNS therapeutics.

Global and Local Efforts to Take Action Against Hepatitis
Lindsey Hiebert and James Amugsi
In this interview, we explore global and local efforts to combat viral hepatitis with Lindsey Hiebert, Deputy Director of the Coalition for Global Hepatitis Elimination (CGHE), and James Amugsi, a Mandela Washington Fellow and Physician Assistant at Sandema Hospital in Ghana. Together, they provide valuable insights into the challenges, successes, and the importance of partnerships in the fight against hepatitis.

Addressing Important Cardiac Biology Questions with Shotgun Top-Down Proteomics
In this interview conducted at Pittcon 2024, we spoke to Professor John Yates about capturing cardiomyocyte cell-to-cell heterogeneity via shotgun top-down proteomics.

Newsletters you may be interested in
Your AI Powered Scientific Assistant
Hi, I'm Azthena, you can trust me to find commercial scientific answers from News-Medical.net.
A few things you need to know before we start. Please read and accept to continue.
- Use of “Azthena” is subject to the terms and conditions of use as set out by OpenAI .
- Content provided on any AZoNetwork sites are subject to the site Terms & Conditions and Privacy Policy .
- Large Language Models can make mistakes. Consider checking important information.
Great. Ask your question.
Azthena may occasionally provide inaccurate responses. Read the full terms .
While we only use edited and approved content for Azthena answers, it may on occasions provide incorrect responses. Please confirm any data provided with the related suppliers or authors. We do not provide medical advice, if you search for medical information you must always consult a medical professional before acting on any information provided.
Your questions, but not your email details will be shared with OpenAI and retained for 30 days in accordance with their privacy principles.
Please do not ask questions that use sensitive or confidential information.
Read the full Terms & Conditions .
Provide Feedback
- Collections
- Diabetes Vic Clinic
- Book a Program
- phone-solid
- Member Login
- Donate Donate
- Languages Shape

1300 437 386
NDSS Helpline
1800 637 700
Member Hub Login
Please login to enjoy your exclusive member hub!
Username or Email Address
Forgot your password?
Not a member? JOIN NOW to access exclusive member benefits!
Having trouble? Visit our Online Help section
Find out other ways to get involved
World-first trial offers new hope for type 1 diabetes
On this page, more blogs dropdown.
- Diabetes management
- Physical activity

Research breakthrough
Researchers at St Vincent’s Institute of Medical Research (SVI) have shown that a commonly used rheumatoid arthritis drug can slow down the progression of type 1 diabetes.
The world-first human trial, led by SVI’s Professors Thomas Kay and Helen Thomas, showed that a drug called baricitinib can safely preserve the body’s own insulin production and slow down the progression of type 1 diabetes in people who start the treatment within 100 days of diagnosis.
Introducing Professor Kay
“When type 1 diabetes is first diagnosed there is a large number of insulin-producing cells still present,” Professor Kay explains.
“We wanted to see if we could protect further destruction of these cells by the immune system. We showed that baricitinib is safe and effective at slowing the progression of type 1 diabetes in people who have been recently diagnosed.”
This groundbreaking research shows promise as the first disease-modifying treatment of its kind for type 1 diabetes that can be delivered as a tablet.
“It is tremendously exciting for us to be the first group anywhere in the world to test the efficacy of baricitinib as a potential type 1 diabetes treatment,” Professor Kay says.
“Up until now, people with type 1 diabetes have been reliant on insulin delivered via injection or infusion pump.
Our trial showed that, if started early enough after diagnosis, and while the participants remained on the medication, their production of insulin was maintained. People with type 1 diabetes in the trial who were given the drug required significantly less insulin for treatment.”
Management of the lifelong autoimmune disease is incredibly burdensome and requires careful glucose monitoring and insulin injections (or use of a pump) day and night to stay alive.
Until insulin’s discovery more than 100 years ago, type 1 diabetes was a fatal condition. While insulin is lifesaving, it can be dangerous if too much or too little is delivered. Diabetes also comes with a higher risk of long-term complications, including heart attack and stroke, vision impairment, kidney disease and nerve damage.
“We are very optimistic that this treatment will become clinically available. This would be a huge step-change in how type 1 diabetes is managed and we believe it shows promise as a fundamental improvement in the ability to control type 1 diabetes,” says Professor Helen Thomas, preclinical lead on the trial.
The clinical trial was funded by Juvenile Diabetes Research Foundation (JDRF), the leading type 1 diabetes research, advocacy, and community programs organisation. Partners included The Royal Melbourne Hospital, St Vincent’s Hospital Melbourne, The Royal Children’s Hospital and The Women’s and Children’s Hospital in Adelaide.
For more information about participating in diabetes research, please click here .
To donate to diabetes research, visit our Donate page . Article published February 2024.
Latest Blogs Articles

This Diabetes Drug Could Lower Your Risk Of Dementia, New Research Says
It's the 'gold standard' in treating congestive heart failure, too.

There has been a lot of chatter over the past year about the positive impacts of diabetes medications—like Ozempic —on a slew of serious health conditions . Now, there’s a new type of prescription joining the conversation—and it may lower your chances of getting dementia .
A recent study found that a class of medications called SGLT-2 inhibitors—which does not include Ozempic or similar drugs like Zepbound —significantly lowered the risk of dementia in people with type 2 diabetes.
Taking a diabetes medication to lower your risk of dementia seems like a big leap, but people with the condition are already at a higher risk of developing it. Here’s what you need to know about the latest findings, plus what’s behind this link.
Meet the experts : Pouya Shafipour, MD , a board-certified family and obesity medicine physician at Providence Saint John’s Health Center in Santa Monica, California. Jamie Alan, PhD , an associate professor of pharmacology and toxicology at Michigan State University.
Why are people with diabetes at a higher risk of dementia?
There is a known link between type 2 diabetes and dementia, with data suggesting that people with type 2 diabetes have a 50 percent greater risk of losing cognitive function. The association is most strongly tied to vascular dementia, which refers to "changes to memory, thinking, and behavior resulting from conditions that affect the blood vessels in the brain," per the National Institute on Aging .
This link is still under investigation, according to the American Heart Association (AHA). But people with type 2 diabetes seem to be at a higher risk if their blood sugar isn’t well-managed.
Research suggests that having too much glucose in the body may increase production of beta-amyloid, which proteins that clump together to form plaques in the brain that have been linked to Alzheimer's disease.
What did the study find?
The study, which was published in BMJ on August 28, analyzed data from the Korean National Health Insurance Service database, which included 110,885 people with type 2 diabetes between the ages of 40 and 69. All of those patients were either taking oral diabetes medications SGLT-2 or DPP-4 inhibitors for their condition. SGLT-2 helps the kidneys remove excess glucose from the body through urine, while DPP-4 works by deactivating the two main hormones that regulate blood sugar levels.
After 670 days, there were 1,172 new diagnoses of dementia among the study participants. When compared with people who took DPP-4 inhibitors, those who took SGLT-2 inhibitors had a 35 percent lower chance of developing dementia. Not only that, they had a 52 percent lower risk of vascular dementia, and a 39 percent lower risk of Alzheimer’s dementia.
People who took SGLT-2 inhibitors for longer periods of time seemed to have the best results. “SGLT-2 inhibitors might prevent dementia, providing greater benefits with longer treatment,” the researchers concluded in the study.
How do SGLT2 inhibitors work?
SGLT-2 inhibitors are a class of medications that treat type 2 diabetes. The medications help keep the kidneys from reabsorbing too much glucose and allow you to pee it out instead of circulating that glucose back into your blood, Alan explains.
“They are on the gold standard treatment list for congestive heart failure, too,” she says.
Why did the drug lower dementia risk?
This study didn’t explore why people had a lower risk of dementia on SGLT-2 inhibitors—it simply found a link. However, it may simply be that people on these medications had better blood sugar control, says Pouya Shafipour, MD , a board-certified family and obesity medicine physician at Providence Saint John’s Health Center in Santa Monica, California.
“Anything that stabilizes or lowers blood sugar and lowers a prolonged hyperglycemic state will help with this,” he says. “SGLT-2 has been found to be very helpful with that.” (Hyperglycemic means high blood sugar, in case you're not familiar with the term.)
Jamie Alan, PhD , an associate professor of pharmacology and toxicology at Michigan State University, agrees. “Lowering blood sugar would theoretically help dementia, particularly vascular dementia,” she says. “However, there is a lot about these drugs that we don’t know.”
Do other diabetes drugs (like Ozempic) have a similar effect?
It’s not clear yet. However, initial research conducted on semaglutide (the active ingredient in Ozempic) and other forms of GLP-1 drugs (the class of medications that Ozempic belongs to) has found that they may lower the risk of dementia.
One study published in December 2022 found that semaglutide lowered the risk of dementia in patients with type 2 diabetes. Another study found that fellow GLP-1 medication liraglutide may protect against dementia.
Are the results applicable to Americans?
This particular study was conducted on people in Korea—not America—and those are two different populations of people. However, experts say these results will likely apply to Americans, too.
How can people with diabetes lower their dementia risk?
It’s too soon to say that you should start taking an SGLT-2 inhibitor to lower your risk of dementia if you have diabetes. However, doing your best to control your blood sugar is often your best bet for lowering your risk of dementia, Shafipour says. That can include doing things like exercising regularly , eating a healthy diet that’s lower in refined carbohydrates, getting enough sleep , and doing your best to manage your stress , he says.
And, if you’re doing all of that and still struggling with blood sugar management, it may be time to rope in your doctor to talk about next steps.
How To Cope With Health Anxiety, Per Psychologists

How to Stay Safe During The Deli Meat Recall

Why Being Flexible May Help You Live Longer

How To Tell If You Have ‘Popcorn Brain’ Or ADHD

Best Electric Razors For Women In 2024, Per Derms

These Herbal Supplements Linked To Liver Damage

Learned Helplessness Can Hold You Back In Life

All About Kamala Harris' Diet And Workouts

How To Use Menstrual Cups And Discs, Per Experts

A Beginner’s Guide To The ‘Hara Hachi Bu’ Method

How To Do A Breast Self-Exam At Home, Per MDs

The Best Daily Supplements For Women Over 50
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Diabetes articles from across Nature Portfolio
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance (cells do not respond to insulin; type 2 diabetes).
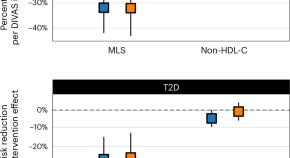
Blood lipid profiling indicates that dietary fat quality is associated with cardiometabolic risk
Dietary guidelines advise substituting saturated fats with unsaturated fats. We used detailed blood fat composition profiling in diet trials and population studies to confirm that a moderate high-fat diet from plant oils is better for metabolism and heart health than a diet with similar fat levels from animal sources.
New insights into the regulation of GIPR signalling
Two recent studies have unravelled novel modes of glucose-dependent insulinotropic polypeptide receptor (GIPR) signalling regulation. Kizilkaya et al. characterized the effect of changes in β-arrestin 2 coupling with naturally occurring GIPR coding variants, whereas Regmi et al. investigated GIPR expression profiles and functional regulation in adipocytes.
- Yusman Manchanda
- Alejandra Tomas
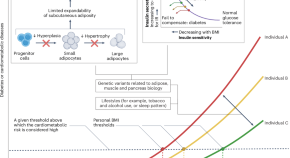
Insights into optimal BMI from the GlasVEGAS study
A human experiment confirms the higher susceptibility of South Asians to adverse metabolic consequences with weight gain compared with white Europeans, which is attributed to underlying differences in muscle and adipose biology.
- Chun-Kwan O
- Juliana C. N. Chan
Related Subjects
- Diabetes complications
- Diabetes insipidus
- Gestational diabetes
- Type 1 diabetes
- Type 2 diabetes
Latest Research and Reviews
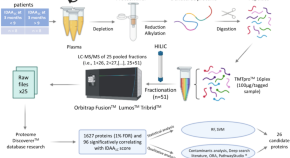
Plasma proteomics in children with new-onset type 1 diabetes identifies new potential biomarkers of partial remission
- Olivier G. Pollé
- Sébastien Pyr dit Ruys
- Philippe A. Lysy
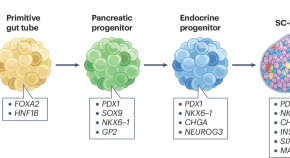
Harnessing cellular therapeutics for type 1 diabetes mellitus: progress, challenges, and the road ahead
Type 1 diabetes mellitus affects 8.5 million people globally and is characterized by autoimmune destruction of pancreatic β cells. This Review discusses cell replacement therapies for T1DM and outlines the challenges and future directions
- Alessandro Grattoni
- Gregory Korbutt
- Paul de Vos
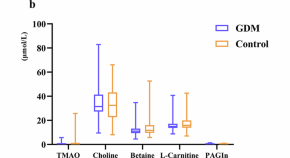
Association of maternal gut microbial metabolites with gestational diabetes mellitus: evidence from an original case-control study, meta-analysis, and Mendelian randomization
- Mengxin Yao
- Xiaoyan Zhu
Finerenone in Heart Failure and Chronic Kidney Disease with Type 2 Diabetes: the FINE-HEART pooled analysis of cardiovascular, kidney, and mortality outcomes
This participant-level pooled analysis of the three phase III trials that have tested the non-steroidal mineralocorticoid receptor antagonist finerenone in patients with heart failure and chronic kidney disease with type 2 diabetes provides an integrated view of the mortality, cardiovascular and renal effects of this treatment. Editor recognition statement: Primary Handling editor: Michael Basson, in collaboration with the Nature Medicine team.
- Muthiah Vaduganathan
- Gerasimos Filippatos
- Scott D. Solomon
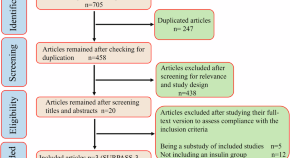
Tirzepatide outcompetes long-acting insulin in managing type 2 diabetes: a meta-analysis of three phase 3 randomized controlled trials
- Razieh Mohammad Jafari
- Mohammad Poursalehian
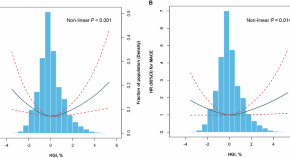
Hemoglobin glycation index and cardiovascular outcomes in patients with diabetes and coronary artery disease: insights from a large cohort study
- Zhangyu Lin
News and Comment
GLP1 agonists: current and future landscape of clinical trials for patients with metabolic dysfunction
Guidelines currently recommend organ-specific management of obesity-related complications. Agents that agonize glucagon-like peptide 1 receptors, including those that co-agonize other anorexigenic hormone receptors, lead to substantial weight loss and benefits in people with varying obesity-related complications, with further trials underway. These medications enable cause-specific management of obesity complications.
- Jonathan Goldney
- Melanie J. Davies

How rival weight-loss drugs fare at treating obesity, diabetes and more
Wegovy, Zepbound and similar medications all lead to metabolic improvements, but scientists are starting to unpick the differences between them.
- Mariana Lenharo
The promising potential of gene therapy for diabetes mellitus
Gene therapy holds tremendous promise for treating a wide range of hereditary and acquired diseases by delivering exogenous therapeutic nucleotide sequences into specific cells or tissues. Recent advances support the notion that gene therapy could offer a long-term cure for diabetes mellitus, something that current conventional pharmacotherapies cannot achieve.
- Stefan R. Bornstein
- J. Fraser Wright
- Charlotte Steenblock
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Diabetic Attacks and Emergencies
Understanding blood sugar, diabetic ketoacidosis, hypoglycemia, hyperglycemia, increased risk of infections, diabetic coma, preeclampsia, heart attack or stroke.
A diabetic attack occurs when your blood sugar spikes too high or drops too low. This can cause a medical emergency.
A number of different conditions and factors can lead to a diabetic emergency, including ketoacidosis , hyperglycemia , and hypoglycemia . Each of these needs to be handled in a specific way to reduce the risk of long-term consequences.
This article explains types of emergencies that can result from diabetic attacks, their symptoms, and treatment options. It also covers possible complications and how to prevent attacks and problems.
Diabetes is a chronic condition where the blood sugar level is too high. Insulin, a hormone produced by the pancreas , removes sugar from the blood and moves it into cells for the body to use. In people with type 1 diabetes , their pancreas doesn’t make any insulin; in those with type 2 diabetes , it doesn’t make enough.
Having high blood sugar for a long period of time puts people with diabetes at risk for other health problems, such as kidney disease, heart disease , stroke , and nerve damage.
A diabetic emergency happens when blood sugar is too high or too low for too long. This is a life-threatening condition that requires immediate medical treatment. There are a few types of diabetic emergencies, and some conditions may increase the risk of a diabetic emergency.
Diabetic ketoacidosis (DKA) occurs when the body begins burning fat, instead of sugar, for fuel. This happens when there isn’t enough insulin to deliver sugar to cells for energy.
To make up for this, the liver begins breaking down fat too quickly for the body to process. This can lead to a buildup of ketones (a type of acid) in the blood, which can become poisonous.
Symptoms of DKA can include:
- Rapid breathing
- Flushed face
- Nausea, vomiting, or abdominal pain
- Decreased alertness
- Frequent urination or thirst that lasts for a day or more
- Dry skin or mouth
- Muscle stiffness or aches
- Dehydration
- Fruity breath
DKA is most common in individuals with type 1 diabetes. It can sometimes be the first sign of type 1 in those who are not diagnosed. Causes of DKA in type 1 diabetes include infection, injury, serious illness, missed insulin doses, or stress due to surgery.
DKA is less common in people with type 2 diabetes. If it occurs, it is typically less severe. Causes of DKA in type 2 diabetes include uncontrolled high blood sugar for a long period of time, missing medicine doses, or a severe illness or infection. It can also be a side effect of some medications for diabetes, such as sodium-glucose transport protein 2 (SGLT2) inhibitors.
When you eat too much sugar, the excess is stored in the muscles and liver. When blood sugar decreases, the liver releases what it has stored, raising the amount of sugar in the blood. For some, especially those with diabetes, their blood sugar doesn’t go up enough and is below 70 mg/dL, causing hypoglycemia, or low blood sugar.
Possible symptoms of hypoglycemia include:
- Fast breathing
- Sweating or chills
- Fast heartbeat
- Lightheadedness or dizziness
- Irritability
- Color draining from the skin
- Blurred vision
- Tingling or numbness in the lips, tongue, or cheeks
- Coordination problems
Hypoglycemia can happen to anyone, but for people with diabetes, hypoglycemia can occur as a side effect of the medicine they’re taking. Eating foods high in carbohydrates usually helps raise your blood sugar to normal levels.
If hypoglycemia happens too often, they need to consult with their healthcare provider to see if they need to change their treatment plan.
Hyperglycemia is blood glucose greater than 125 mg/dL while fasting, which is defined as not eating for at least eight hours.
It can occur in people with diabetes if they’re eating too many carbohydrates, taking their medicine incorrectly, or if their medication is not as effective as it should be.
Stress and the dawn phenomenon (a surge of hormones that leads to high blood sugar in the morning), could also lead to hyperglycemia.
Symptoms of hyperglycemia can include:
- Increased urination or thirst
- Slow-healing cuts and sores
Hyperglycemic hyperosmolar syndrome (HHS) can occur if you have a high blood sugar level for a long time. Signs of HHS can include:
- Blood sugar over 600 mg/dL
- Extreme thirst or dry mouth
- Confusion, hallucinations, drowsiness, or passing out
- Fever over 100.4 degrees F
- Weakness or paralysis on one side of the body
- Frequent urination
HHS usually develops in people who do not have their type 2 diabetes under control and who have an infection, stopped taking their medications, have a heart attack or stroke, or take medicine that can cause this condition, such as steroids and diuretics.
High blood sugar can negatively affect the immune system. It can lower the ability of white blood cells to come to the site of an infection and kill what is causing the infection. Nerve damage and difficulty breaking down and storing fats can contribute to an increased risk of infection.
People with type 1 or type 2 diabetes are vulnerable to infections that can become life-threatening, including:
- Fungal infections, such as jock itch, athlete’s foot, ringworm , and vaginitis
- Urinary tract infections
- Bacterial infections of the skin and soft tissue that won’t heal
Signs of infection can include fever, chills, sore throat or mouth sores, redness or swelling, or pain with urination.
A diabetic coma , where a person passes out due to extremely low or high blood sugar, is an emergency that requires immediate medical attention. Extreme hypoglycemia or hyperglycemia can cause a diabetic coma, so symptoms of these two conditions could be warning signs of this diabetic emergency.
Other circumstances can also increase the risk of diabetic coma, such as:
- Surgery or other bodily trauma
- Illness or infection
- Drinking alcohol
- Skipping insulin doses
- Poor diabetes management
Diabetic ketoacidosis and hypoglycemia are more likely to cause a diabetic coma in those with type 1 diabetes, while HHS places people with type 2 diabetes more at risk of this condition.
When to Call Your Healthcare Provider
You should call your healthcare provider or 911 if you have diabetes and the following:
- Your blood sugar is 300 mg/dL or higher two times in a row for an unknown reason.
- You have low blood sugar that has not come up after three treatments.
Preeclampsia is pregnancy-induced high blood pressure ( hypertension ) and liver or kidney damage. It often occurs after the 20 th week of pregnancy. The risk of preeclampsia is two to four times higher among people with type 1 or type 2 diabetes. Gestational diabetes , a type of diabetes that occurs during pregnancy, also increases your risk of developing preeclampsia.
The exact cause of preeclampsia is unknown. It is estimated to occur in about 3% to 7% of all pregnancies.
Women with preeclampsia often do not feel sick, but symptoms in the early stages could include:
- Swelling of the hands and face or eyes
- Sudden weight gain over one to two days or more than two pounds a week
- Headache that does not go away or becomes worse
- Trouble breathing
- Belly pain on the right side, below the ribs
- Not urinating very often
- Nausea and vomiting
- Vision changes, such as temporary blindness, seeing flashing lights or spots, sensitivity to light, and blurry vision
- Feeling lightheaded or faint
Even when diabetes is controlled, high blood sugar can still damage the blood vessels and nerves of the heart over the years. The longer you have diabetes, the higher the chances that you will develop heart disease . This increases the risk of heart attack or stroke.
Signs of a heart attack can include:
- Pain or pressure in your chest that lasts longer than a few minutes or goes away and returns
- Pain or discomfort in one or both arms, or the shoulders, back, neck, or jaw
- Shortness of breath
- Sweating or lightheadedness
- Feeling extreme fatigue
- Indigestion or nausea
Women are more likely to experience nausea or vomiting, back or jaw pain, and shortness of breath as heart attack symptoms.
Signs of a stroke are:
- Sudden numbness or weakness on one side of the body
- Trouble seeing or walking
- Sudden severe headaches with no known cause
- Confusion, difficulty speaking or understanding speech
If you experience any of these symptoms, call 911 immediately.
To avoid a diabetic emergency, you must manage your diabetes as well as possible. Check your blood sugar often, and get into the habit of recognizing the early signs that levels are rising or dropping toward a dangerous range.
Other tips to prevent a diabetic emergency include:
- Eat regularly and avoid foods that are processed or have added sugar
- Stay active and exercise regularly
- Take medications as prescribed
It’s also a good idea to carry snacks that you can eat to quickly get sugar into your blood to treat hypoglycemia. These might include raisins, candy, or glucose tablets.
For hyperglycemia, exercise will lower your blood sugar, but if your blood sugar is above 240 mg/dL, you need to check your urine for ketones. Exercising with a high ketone level will raise your blood sugar even higher.
If you are pregnant, your healthcare provider may recommend that you take daily low-dose aspirin to help prevent preeclampsia and its related complications. It is started between 12 to 28 weeks of pregnancy, but it is best to start before 16 weeks of pregnancy.
Diabetic attacks can be caused by hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar), which can cause medical emergencies. Too little insulin can also cause an emergency condition known as diabetic ketoacidosis. During pregnancy, high blood pressure can also put you at risk for a diabetic attack.
Uncontrolled diabetes results in more than a diabetic attack, though. It also increases susceptibility to infections and puts you at risk for suffering from a diabetic coma.
There are steps you can take to reduce your risk and maintain a stable sugar level.
A Word From Verywell
Managing diabetes and the possibility of diabetic emergencies can feel overwhelming, but these emergencies are largely preventable by keeping your condition under control.
Eating healthy, taking medicines as prescribed, exercising regularly, and recognizing the early signs of rising or falling blood sugar levels can help you keep these emergencies at bay and become prepared in the event that they do occur.
National Institutes of Health. What is diabetes ?
American Diabetes Association. Hypoglycemia (low blood sugar) .
MedlinePlus. Diabetic ketoacidosis .
National Institute of Diabetes and Digestive and Kidney Diseases. Hypoglycemia .
American Diabetes Association. Hyperglycemia (high blood sugar) .
MedlinePlus. Diabetic hyperglycemic hyperosmolar syndrome .
Stoner GD. Hyperosmolar hyperglycemic state . Am Fam Physician ; 96(11):729-736.
Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study . Diabetes Care . 2018;41(3):513-521. doi:10.2337/dc17-2131
Centers for Disease Control and Prevention. Know the signs and symptoms of an infection .
Cleveland Clinic. Diabetic coma .
Weissgerber TL, Mudd LM. Preeclampsia and diabetes . Curr Diab Rep . 2015;15(3):9. doi:10.1007/s11892-015-0579-4
MedlinePlus. Preeclampsia .
National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes, heart disease, and stroke .
American Heart Association. Heart attack symptoms in women .
Centers for Disease Control and Prevention. Stroke signs and symptoms .
American Diabetes Association. Eating well .
U.S. Preventive Services Task Force. Aspirin use to prevent preeclampsia and related morbidity and mortality: U.S. Preventive Services Task Force recommendation statement .
By Carisa Brewster Brewster is a freelance journalist with over 20 years of writing experience specializing in science and healthcare content.

COMMENTS
Type 1 diabetes (also known as diabetes mellitus) is an autoimmune disease in which immune cells attack and destroy the insulin-producing cells of the pancreas. ... Latest Research and Reviews ...
Type 1 diabetes is an autoimmune condition resulting in insulin deficiency and eventual loss of pancreatic β cell function requiring lifelong insulin therapy. Since the discovery of insulin more than 100 years ago, vast advances in treatments have improved care for many people with type 1 diabetes. Ongoing research on the genetics and immunology of type 1 diabetes and on interventions to ...
Partial remission (PR) occurs in only half of people with new-onset type 1 diabetes (T1D) and corresponds to a transient period characterized by low daily insulin needs, low glycemic fluctuations ...
Type 1 diabetes is a chronic autoimmune disease that requires lifelong care including requiring insulin, either through multiple daily injections or continuous infusion using a pump, every day to ...
Both are now adults, and both have Type 1 diabetes. My son was 6 months old when he was diagnosed. And that's when I changed my research plan. And my daughter, who's four years older than my son, became diabetic about 10 years later, when she was 14. When my son was diagnosed, I knew nothing about diabetes and had been working on how frogs ...
Hummel, S. et al. Children diagnosed with presymptomatic type 1 diabetes through public health screening have milder diabetes at clinical manifestation. Diabetologia 66 , 1633-1642 (2023).
In fulminant new-onset type 1 diabetes, intensive insulin therapy is known to be associated with improved glycaemic and microvascular outcomes. ... REJB is funded by the Oxford National Institute for Health and Care Research Biomedical Research Centre, Oxford, UK, through the JDRF/Wellcome Strategic Award (4-SRA-2017-473-A-N; 107212/A/15/Z) and ...
Type 1 diabetes mellitus (T1DM) is a growing global health concern that affects approximately 8.5 million individuals worldwide. ... 43 Research Department, Breakthrough T1D, New York, NY, USA. [email protected]. 44 Vaccine and Immunotherapy Center, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. mark ...
New advances in type 1 diabetes. In table 3 in this review by Subramanian and colleagues (BMJ 2024;384:e075681, doi: 10.1136/bmj-2023-075681, published 26 January 2024), the time in range target for most adults with type 1 diabetes has been corrected to >70%. The article and PDF have been updated.
A new study led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment. The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that ...
"This work was motivated by a major unmet need," said Veiseh, a Rice assistant professor of bioengineering and Cancer Prevention and Research Institute of Texas scholar. "In type 1 diabetes ...
"Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures," said Peter Marks, MD, PhD, director of the FDA's Center for Biologics Evaluation and Research, in a FDA press release about Lantidra. "Today's approval, the first-ever cell therapy to treat patients with type 1 diabetes, provides individuals living with type 1 ...
In clinical investigations, low-dose anti-thymocyte globulin (ATG) treatment significantly (vs placebo) preserved C-peptide secretion and improved glycaemic control in children, as well as adults, with new-onset type 1 diabetes [36-38]. The potential benefits of ATG appear to depend on the dose level and the age of the recipients, and the ...
Some of the targets are already being studied in type 1 diabetes clinical trials of various therapies, but others could potentially be explored in future trials to prevent type 1 diabetes. This large research study with a diverse population has provided important knowledge about the complex type 1 diabetes genetics landscape, revealing new ...
New technologies in type 1 diabetes. Intensive insulin therapy for the management of type 1 diabetes (T1D) was established as the standard of care based on the results of the Diabetes Control and Complication Trial (DCCT), which conclusively demonstrated the benefits of tight glycemic control. 1 However, those who received intensive insulin management were at increased risk for severe ...
Monash University. "Potential target for Type 1 diabetes treatment." ScienceDaily. ScienceDaily, 22 July 2022. <www.sciencedaily.com / releases / 2022 / 07 / 220722082419.htm>. Scientists have ...
Date: April 27, 2023. Source: Rice University. Summary: Scientists identified three biomaterial formulations that could help develop a more sustainable, long-term, self-regulating way to treat ...
Nature Medicine explores the latest translational and clinical research news, with an immunotherapy that delays the onset of type 1 diabetes in high-risk children and adults.
In the field of Type 1 diabetes, research has largely focused on understanding the immune component, but our study argues that the β-cell is a significant player. Our findings suggest that the β ...
Identification of a new player in type 1 diabetes risk. Type 1 diabetes is caused by an autoimmune attack of insulin-producing beta-cells. While genetics and the environment are known to play important roles, the underlying factors explaining why the immune system mistakenly recognize beta-cells as foreign is not known.
The burden of type 1 diabetes remains substantial, and more research is needed to improve the lives of people with type 1 diabetes and to find a cure. To this end, ADA-funded research continues to drive progress by funding research projects topics spanning technology, islet transplantation, immunology, improving transition to self-management ...
The American Diabetes Association funds a productive research portfolio that offers significant progress and hope for improved outcomes for people with type 1 diabetes. Identifying type 1 diabetes before beta cell loss. Dr. Hessner is investigating so-called "biomarkers," which are components in blood or tissue samples that can be measured ...
The top ten priorities will help to guide future type 1 diabetes research in the UK and Ireland to make sure it has the greatest possible benefit for people with the condition. ... The latest Type 1 PSP - which condensed and whittled down nearly 3000 questions submitted by people affected by type 1 to a shortlist of the top ten ...
Type 1 diabetes risk after age 10 differs between boys and girls; A total of 19 adults with type 1 diabetes took part in the study. The research team looked at each participant's glycaemic and cardiovascular responses following interval exercise sessions and continuous exercise sessions.
Type 1 diabetes mellitus (T1DM) can be predicted, and immune therapy can alter the progression of the disease. ... Research Highlight | 22 March 2021. New combination therapy shows promise for ...
Study reveals reduced type 1 diabetes risk in post-10-year-old girls. New research presented at this year's Annual Meeting of the European Association for Study of Diabetes (Madrid, Spain, 9-13 ...
This groundbreaking research shows promise as the first disease-modifying treatment of its kind for type 1 diabetes that can be delivered as a tablet. "It is tremendously exciting for us to be the first group anywhere in the world to test the efficacy of baricitinib as a potential type 1 diabetes treatment," Professor Kay says.
This Diabetes Drug Could Lower Your Risk Of Dementia, New Research Says It's the 'gold standard' in treating congestive heart failure, too. By Korin Miller Published: Sep 04, 2024 1:59 PM EDT
Diabetes describes a group of metabolic diseases characterized by high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes) or by insulin resistance ...
What to Do When a Diabetic Attack or Emergency Strikes